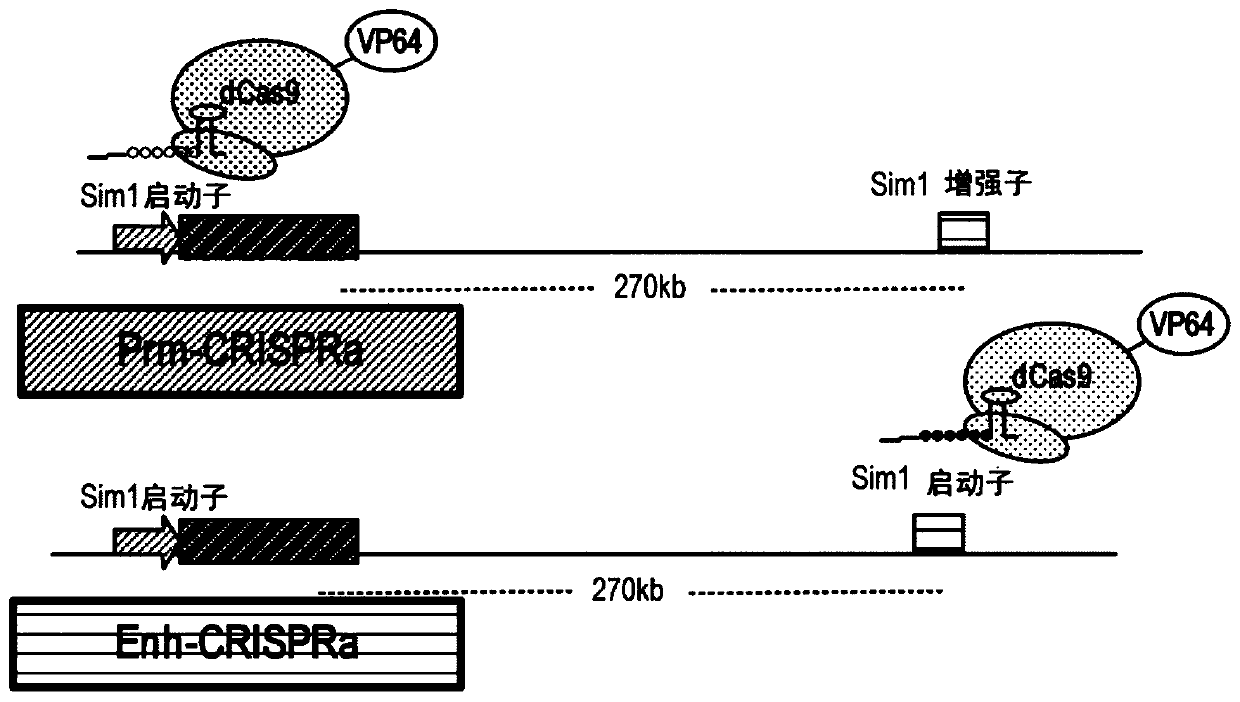
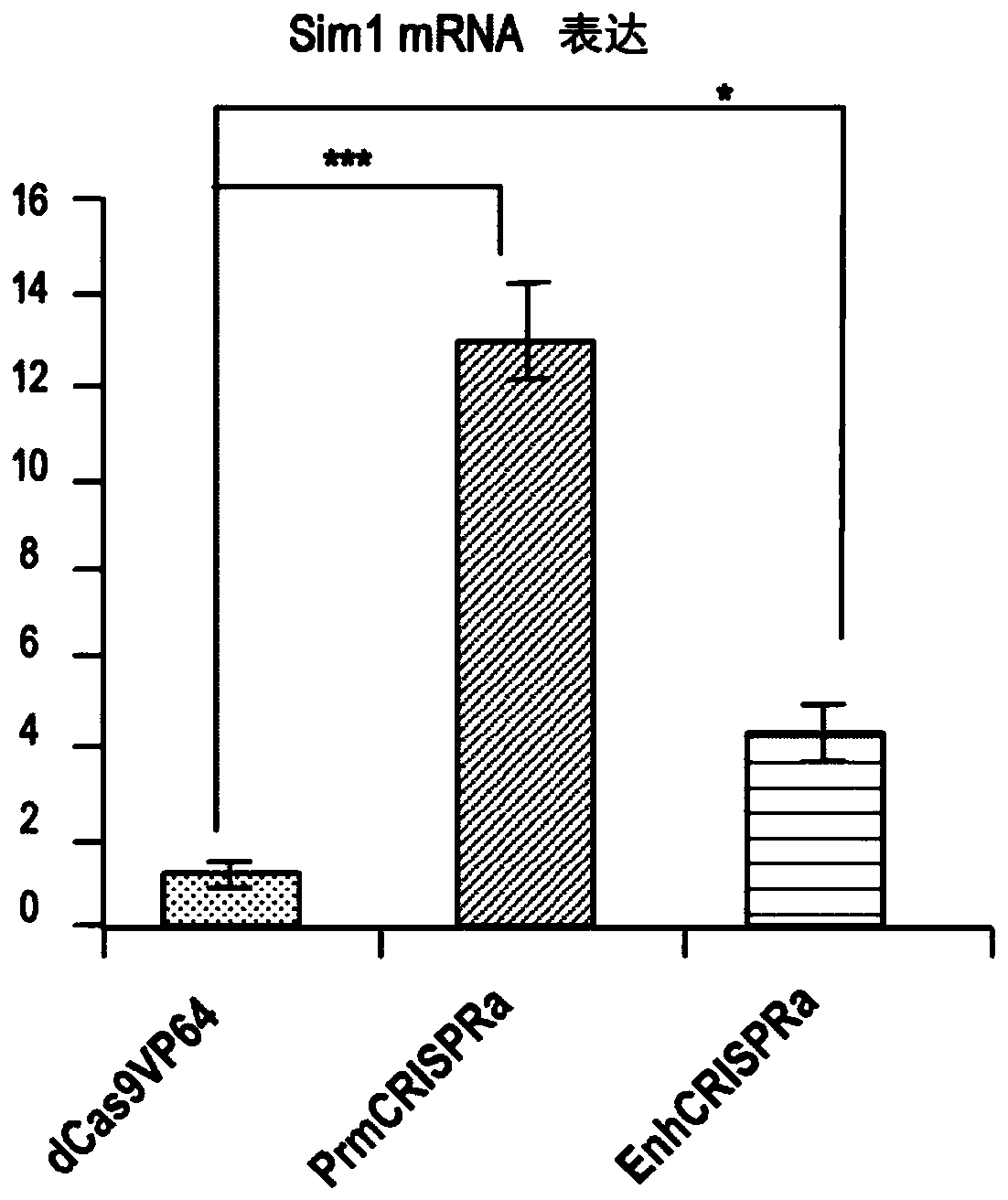
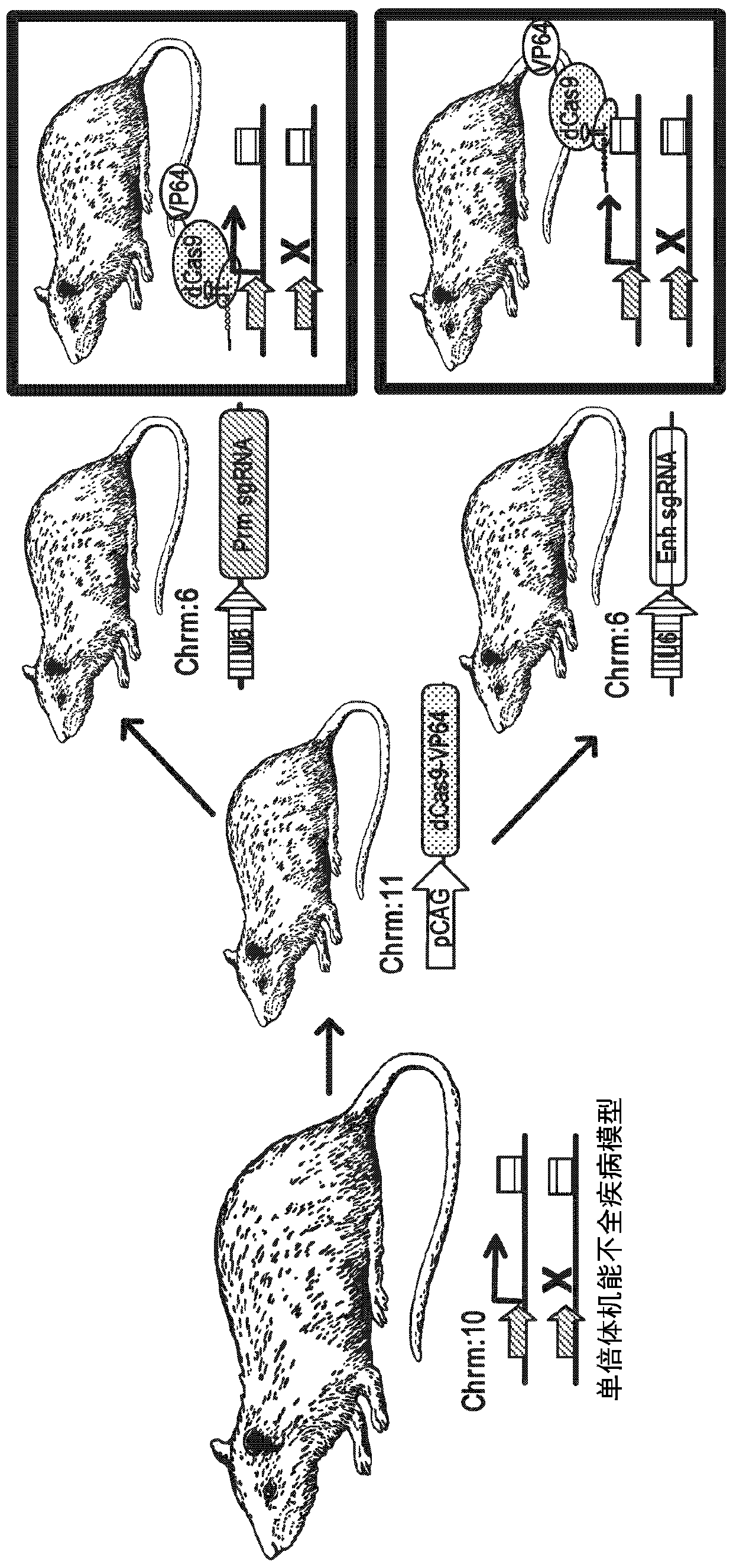
Gene therapy for haploinsufficiency
A dysfunctional, haploid technology, applied in gene therapy, genetic engineering, virus/bacteriophage, etc., can solve the problems of reduced transcript stability, reduced gene transcription, and insufficient gene products to produce wild-type phenotypes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0241] Rescuing obesity from haploid insufficiency
[0242] I. Introduction
[0243] More than 300 genes are known to contribute to human disease due to haploinsufficiency (1, 2), resulting in a wide range of phenotypes including cancer, neurological disorders, developmental disorders, immune disorders, metabolic disorders, infertility, renal disease, limb deformities etc. (1). Large-scale exome sequencing analysis estimated a total of 3230 human genes that may be heterozygous for loss-of-function (LoF) intolerance (3). Gene therapy holds great promise for correcting haploid insufficiency disorders by inserting one or more functionally recombinant copies of a mutated gene. Currently, 2300 gene therapy clinical trials are underway, most of which use adeno-associated virus (AAV) to deliver recombinant genes (4). AAV is a preferred method of gene delivery because of its ability to deliver DNA without integration into the genome, without causing pathogenicity and providing long...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com