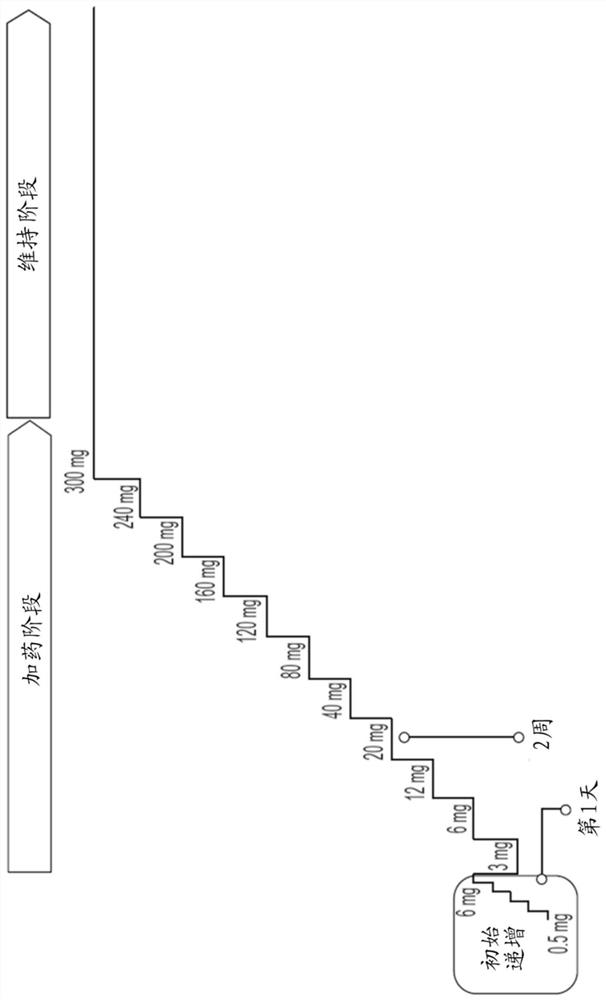
Peanut oral immunotherapy dosing schedule for missed doses
A technology of immunotherapy and dosage, which is applied in drug combination, drug delivery, pharmaceutical formulation, etc., and can solve problems such as missed doses and dosing phases or maintenance phases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Example 1: Continuing Oral Immunotherapy for Peanut Allergy
[0150] Peanut allergy in a patient with a diagnosed peanut allergy was treated by administering a peanut protein pharmaceutical formulation according to an oral immunotherapy schedule. The daily dosage levels for the dosing phase of the schedule were 3 mg, 6 mg, 12 mg, 20 mg, 40 mg, 80 mg, 120 mg, 160 mg, 200 mg, 240 mg and 300 mg peanut protein. Each dose level for the dosing phase was indicated for two weeks of administration, as tolerated. The maintenance phase of the schedule included a daily dose level of 300 mg peanut protein, as tolerated. In the clinic, and under medical supervision, patients undergo initial dose escalation through doses of 0.5 mg, 1 mg, 1.5 mg, 3 mg, and 6 mg of peanut protein with intervals between doses of 20 to 30 minutes.
[0151] The next day, the patient returned to the clinic where she was administered a dose of 3 mg peanut protein, the first dose of the dosing schedule. ...
Embodiment 2
[0153] Example 2: Continuing Oral Immunotherapy for Peanut Allergy
[0154]Peanut allergy in a patient with a diagnosed peanut allergy was treated by administering a peanut protein pharmaceutical formulation according to an oral immunotherapy schedule. The daily dosage levels for the dosing phase of the schedule were 3 mg, 6 mg, 12 mg, 20 mg, 40 mg, 80 mg, 120 mg, 160 mg, 200 mg, 240 mg and 300 mg peanut protein. Each dose level for the dosing phase was indicated for two weeks of administration, as tolerated. The maintenance phase of the schedule included a daily dose level of 300 mg peanut protein, as tolerated. In the clinic, and under medical supervision, patients undergo initial dose escalation through doses of 0.5 mg, 1 mg, 1.5 mg, 3 mg, and 6 mg of peanut protein with intervals between doses of 20 to 30 minutes.
[0155] The next day, the patient returned to the clinic where she was administered a dose of 3 mg peanut protein, the first dose of the dosing schedule. P...
Embodiment 3
[0157] Example 3: Continuation of Oral Immunotherapy for Peanut Allergy
[0158] Peanut allergy in a patient with a diagnosed peanut allergy was treated by administering a peanut protein pharmaceutical formulation according to an oral immunotherapy schedule. The daily dosage levels for the dosing phase of the schedule were 3 mg, 6 mg, 12 mg, 20 mg, 40 mg, 80 mg, 120 mg, 160 mg, 200 mg, 240 mg and 300 mg peanut protein. Each dose level for the dosing phase was indicated for two weeks of administration, as tolerated. The maintenance phase of the schedule included a daily dose level of 300 mg peanut protein, as tolerated. In the clinic, and under medical supervision, patients undergo initial dose escalation through doses of 0.5 mg, 1 mg, 1.5 mg, 3 mg, and 6 mg of peanut protein with intervals between doses of 20 to 30 minutes.
[0159] The next day, the patient returned to the clinic where she was administered a dose of 3 mg peanut protein, the first dose of the dosing schedu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com