Patents
Literature
39 results about "Peanut food allergy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peanut allergy is a type of food allergy to peanuts. It is different from tree nut allergies. Physical symptoms of allergic reaction can include itchiness, hives, swelling, eczema, sneezing, asthma, abdominal pain, drop in blood pressure, diarrhea, and cardiac arrest. Anaphylaxis may occur.
Manufacture of peanut formulations for oral desensitization
The present application relates to a method for managing the development and manufacturing process of a therapeutically effective formulation. Peanut proteins are characterized from peanut flour and encapsulated formulations made using the peanut flour for oral immunotherapy of peanut allergies.
Owner:SOC DES PROD NESTLE SA
Peanut formulations and uses thereof
The present application relates to compositions for oral immunotherapy of peanut allergies. Further, the present application relates to methods for the preparation of the compositions for immunotherapy, and their use in immunotherapy.
Owner:SOC DES PROD NESTLE SA
Supression of ige production by compounds derived from traditional chinese medicine
ActiveUS20160175291A1Suppressing IgE productionInhibiting IgE productionBiocideKetone active ingredientsPeanut food allergyChinese drug
The present invention relates to compounds and formulations derived from traditional Chinese medicine for use for the treatment and / or mediation of food allergies, in particular peanut allergies in humans by altering the activity and / or production of IgE.
Owner:SEAN N PARKER FOUND +1
Methods of oral immunotherapy
ActiveUS20190167785A1Reduce incidenceReduce riskAllergen ingredientsPharmaceutical delivery mechanismPeanut food allergyOral immunotherapy
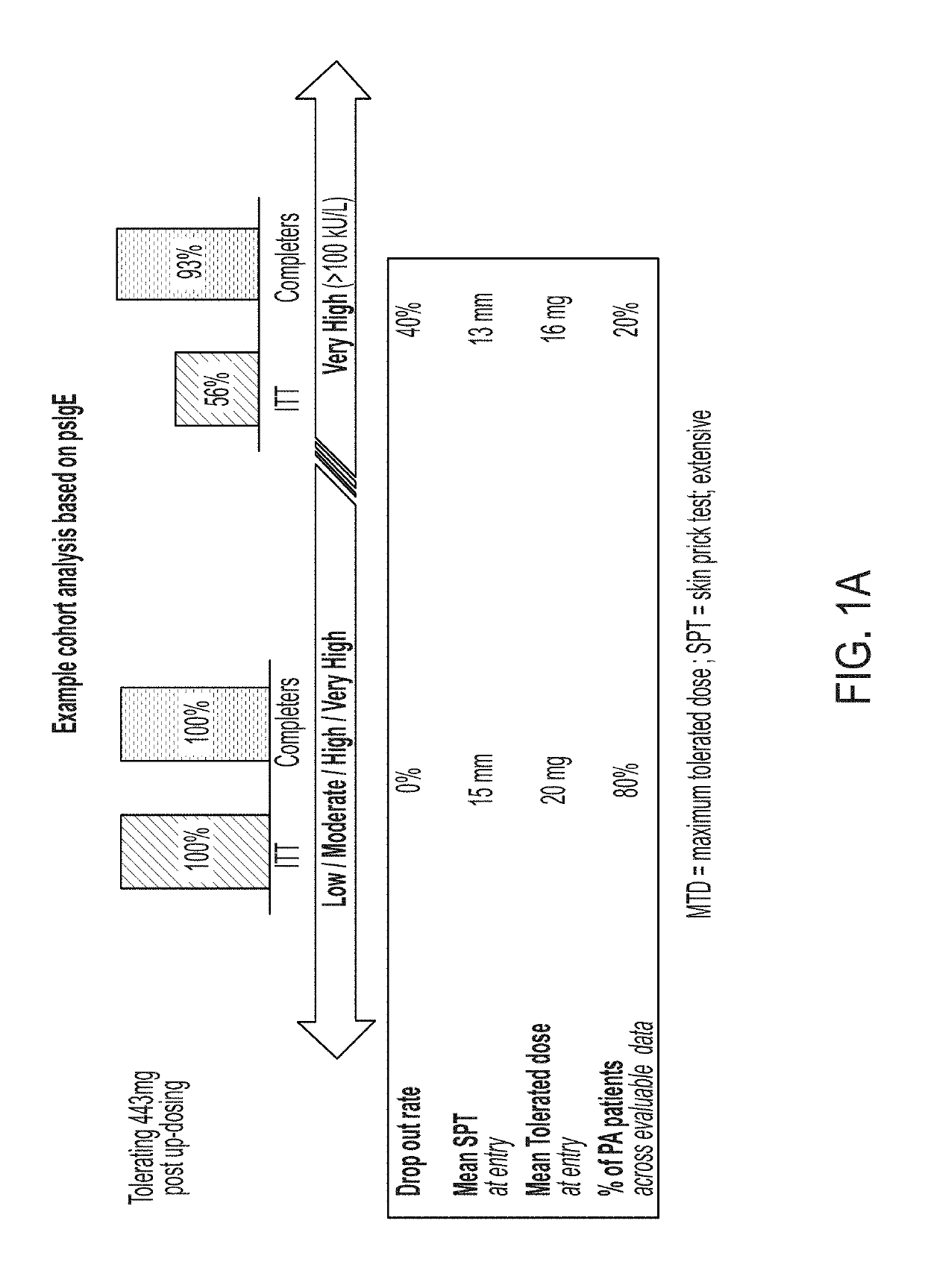
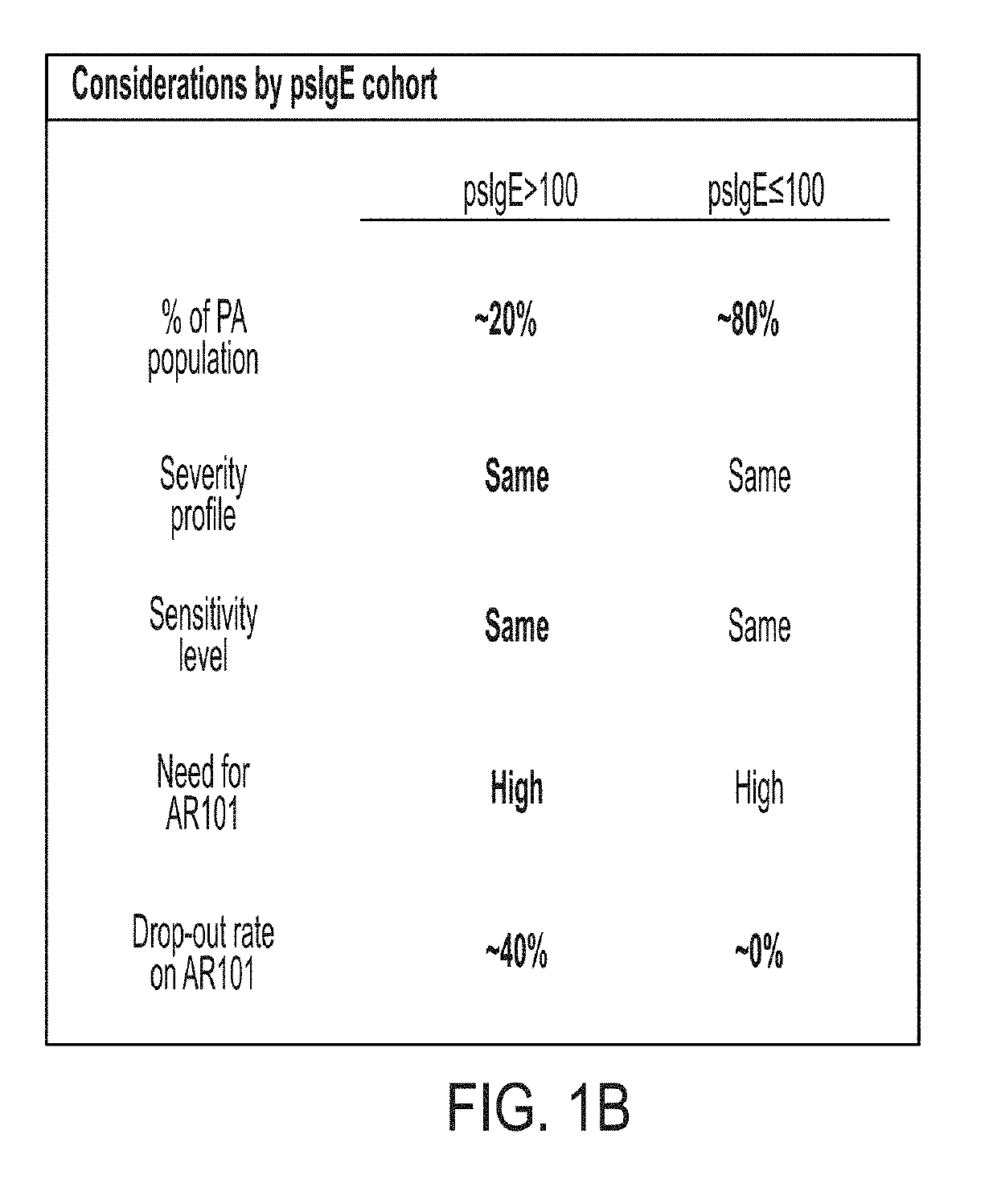
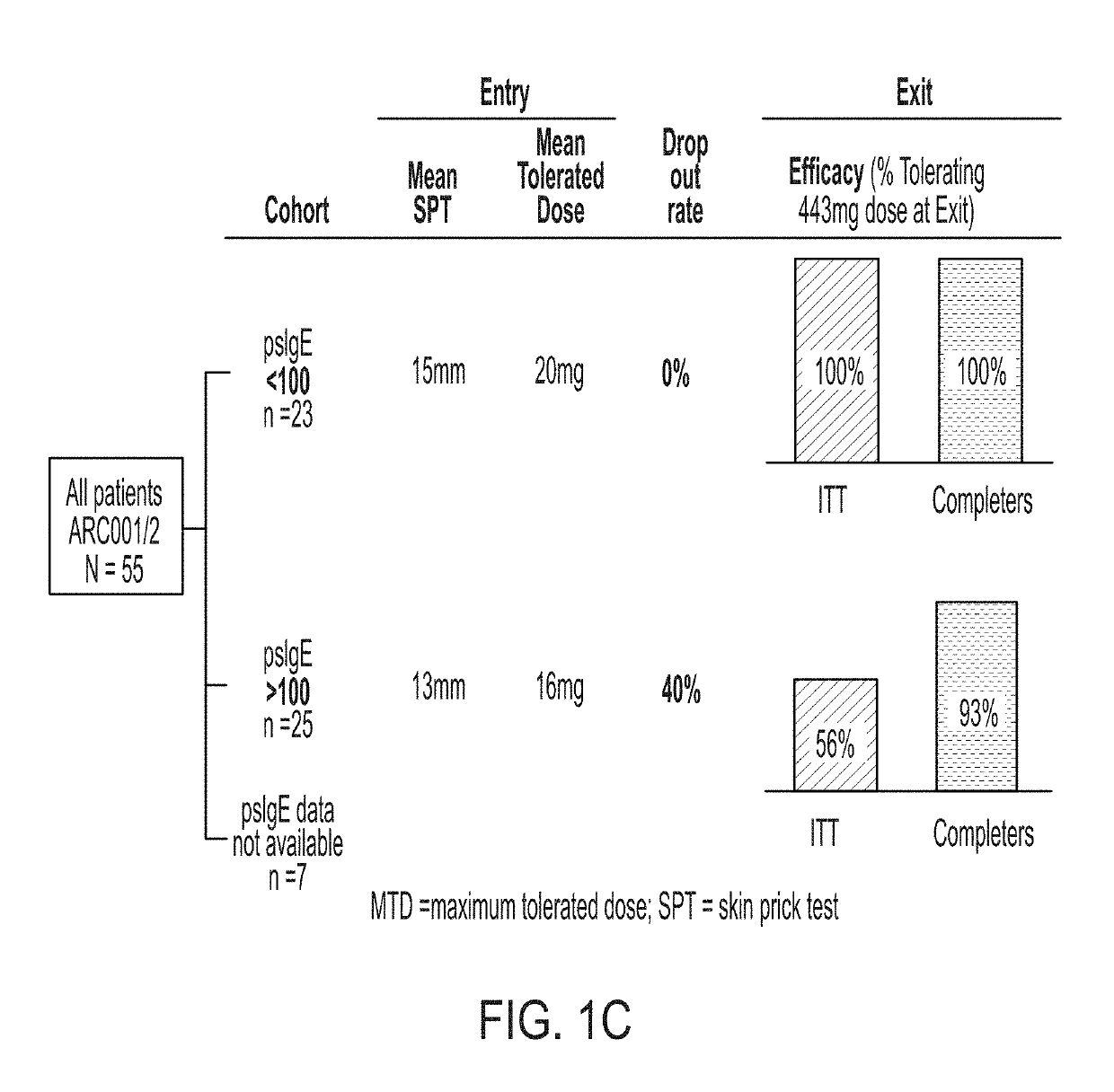
The present disclosure relates to improved oral immunotherapy methods for treating food allergies, and particularly peanut allergy. Biomarkers, such as a level of peanut-specific IgE and / or peanut-specific IgG4s are used by the methods described herein. Such methods include a method of treating a subject for a peanut allergy, methods of assessing the suitability of a treatment for a peanut allergy, a method of evaluating a symptom in a subject during the course of treatment of a peanut allergy, a method of monitoring treatment for a peanut allergy in a subject, a method of reducing the risk or incidence of an adverse event in a subject receiving treatment for a peanut allergy, a method of adjusting a dose of an allergenic peanut composition, and a method of assessing a likelihood of an allergic reaction that requires administration of epinephrine to a subject receiving treatment for a peanut allergy.
Owner:SOC DES PROD NESTLE SA
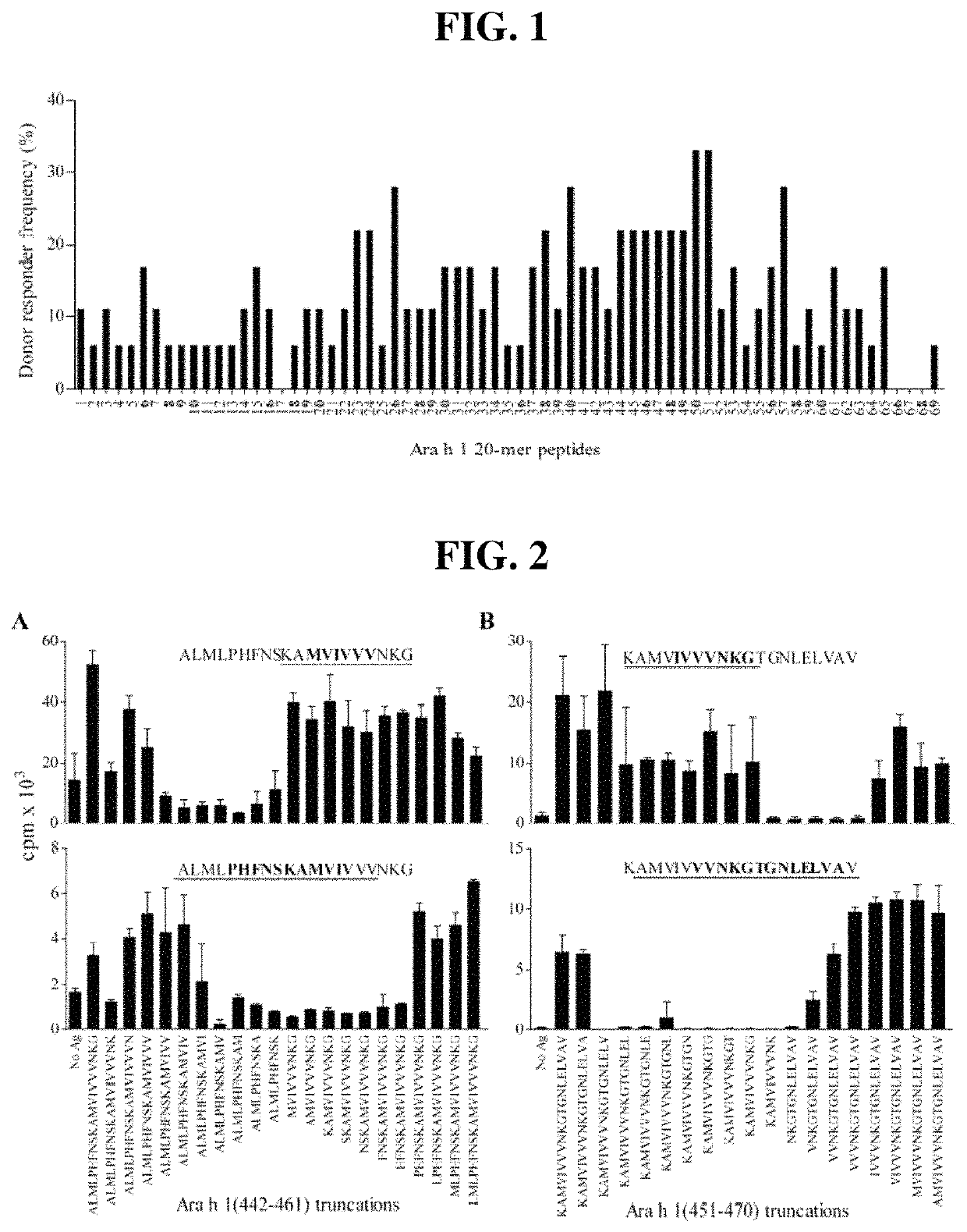
Novel Immunointeractive Molecules and uses Thereof
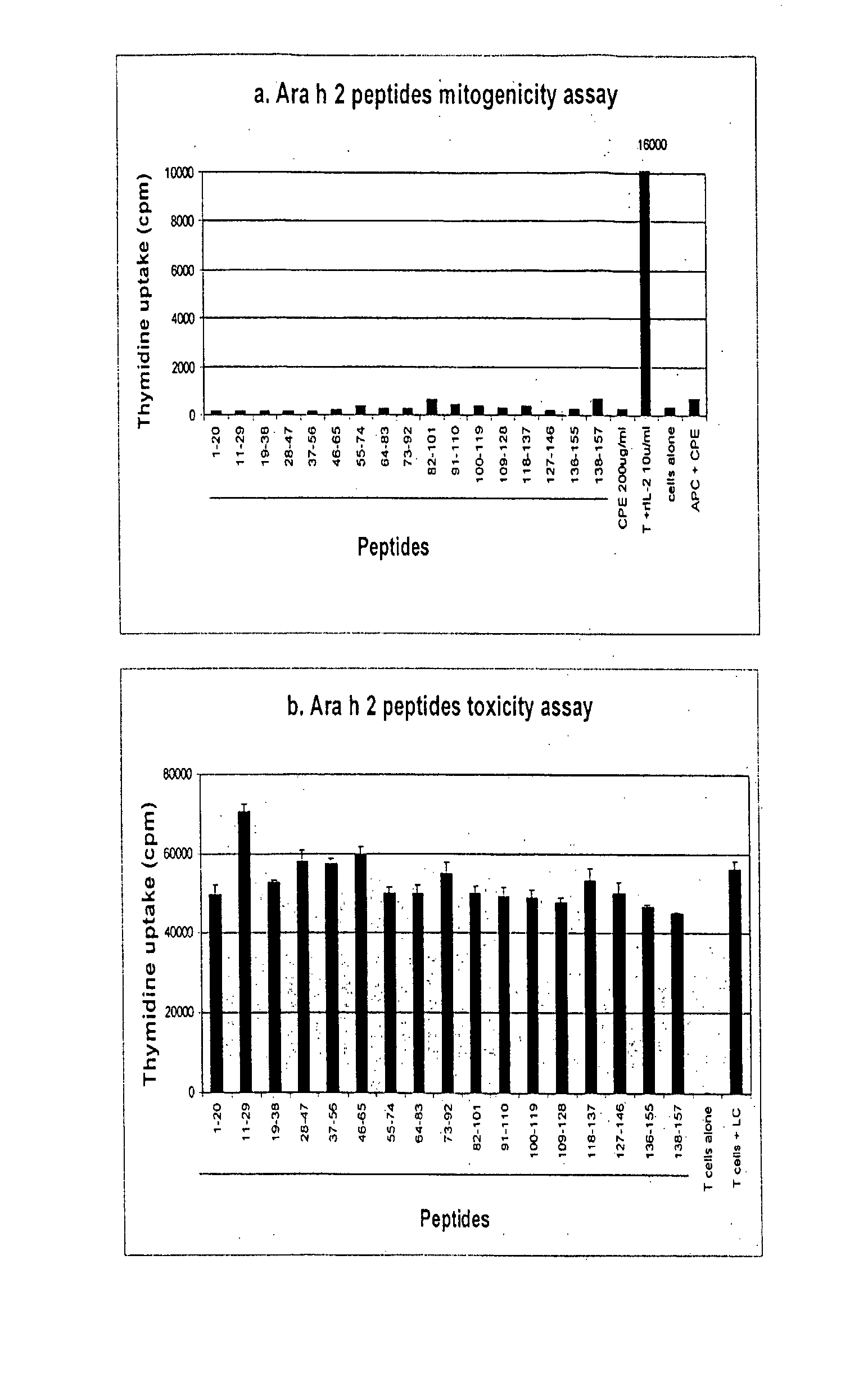
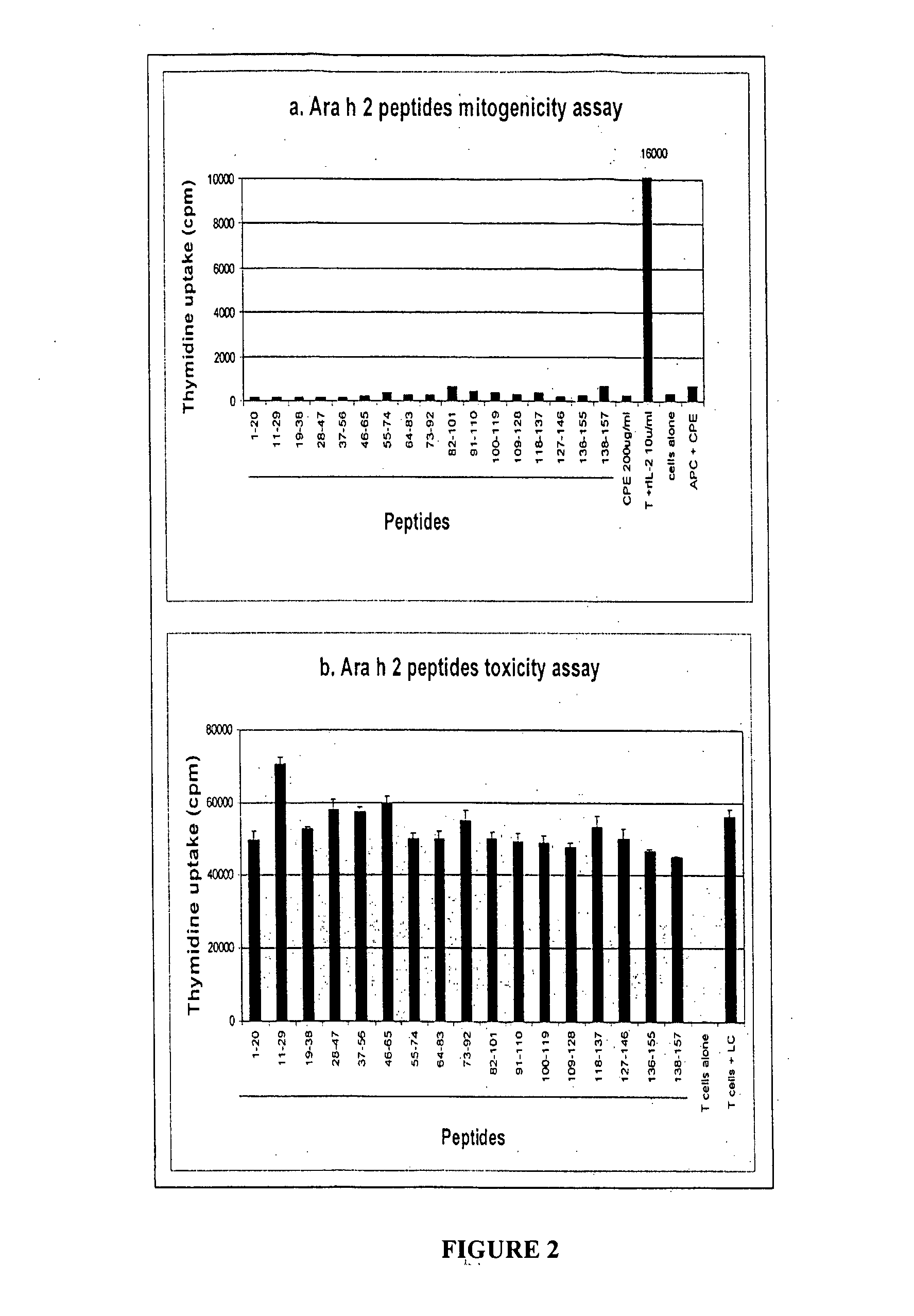
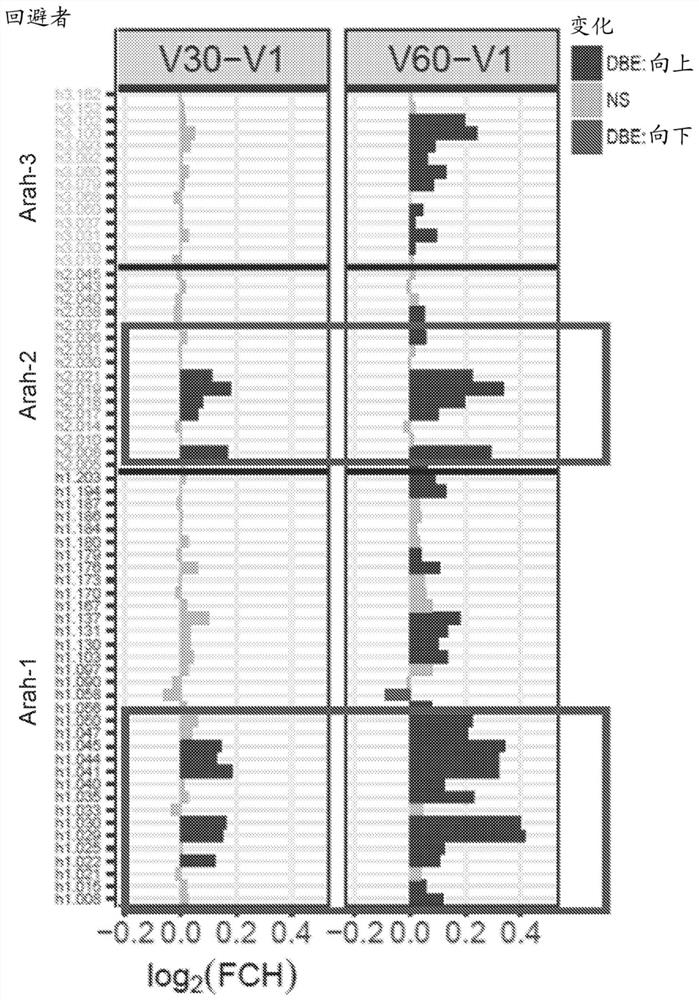
The present invention relates generally to molecules such as peptides, polypeptides and proteins which interact immunologically with T lymphocytes in subjects having peanut allergy, or allergy to other tree nuts, and genetic sequences encoding same. These molecules are preferentially immunointeractive with T cells in subjects having an allergy to the Ara h 2 allergen. The molecules of the present invention are useful in the development of diagnostic, therapeutic and prophylactic agents for conditions characterised by an aberrant, inappropriate or otherwise unwanted immune response to Ara h 2 or derivative or homologue thereof.
Owner:ARAVAX
Peanut allergy treatment
InactiveUS20140010849A1Reduces and eliminates peanut-induced anaphylaxisReduces and eliminates airway inflammationAntibody mimetics/scaffoldsPharmaceutical delivery mechanismPeanut food allergyAllergic reaction
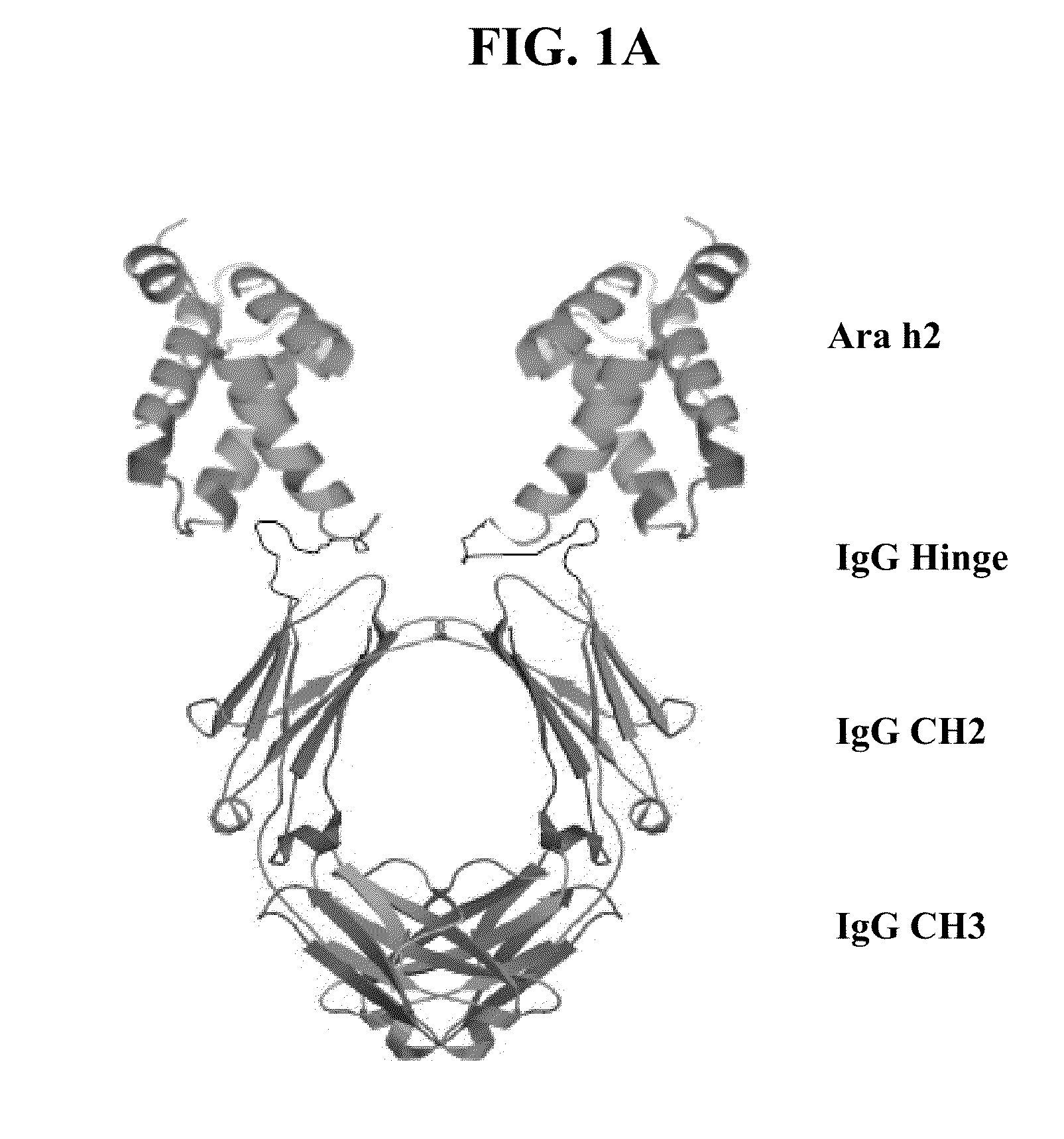
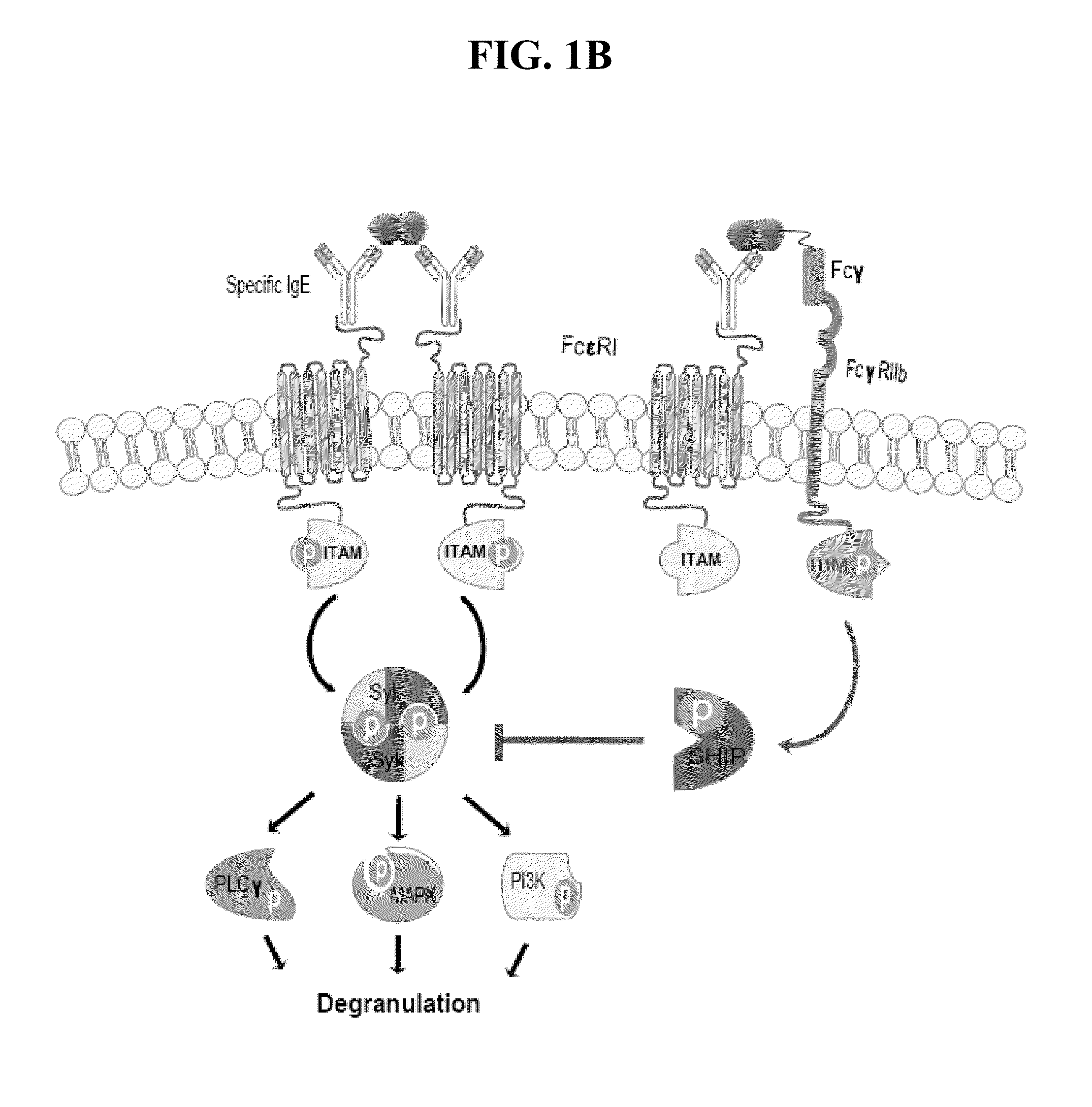
The present invention provides compositions, systems, and methods for preventing and treating allergic reactions to peanut exposure. In certain embodiments, the present invention employs fusion proteins comprising a peanut allergen (or allergenic portion thereof), such as Ara h2, and an Fc protein (e.g., Fcγ1 protein), or functional portion thereof.
Owner:NORTHWESTERN UNIV
Nucleic acids for treatment of peanut allergies
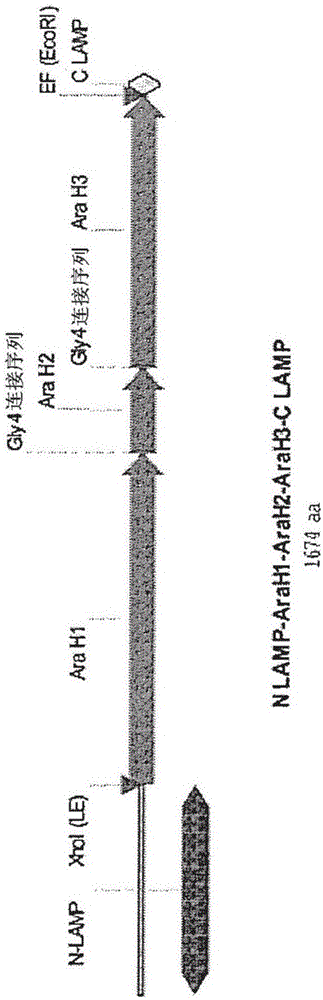
InactiveCN106459994AFusions for enhanced expression stability/foldingWhole-cell/virus/DNA/RNA ingredientsPeanut food allergyEpitope
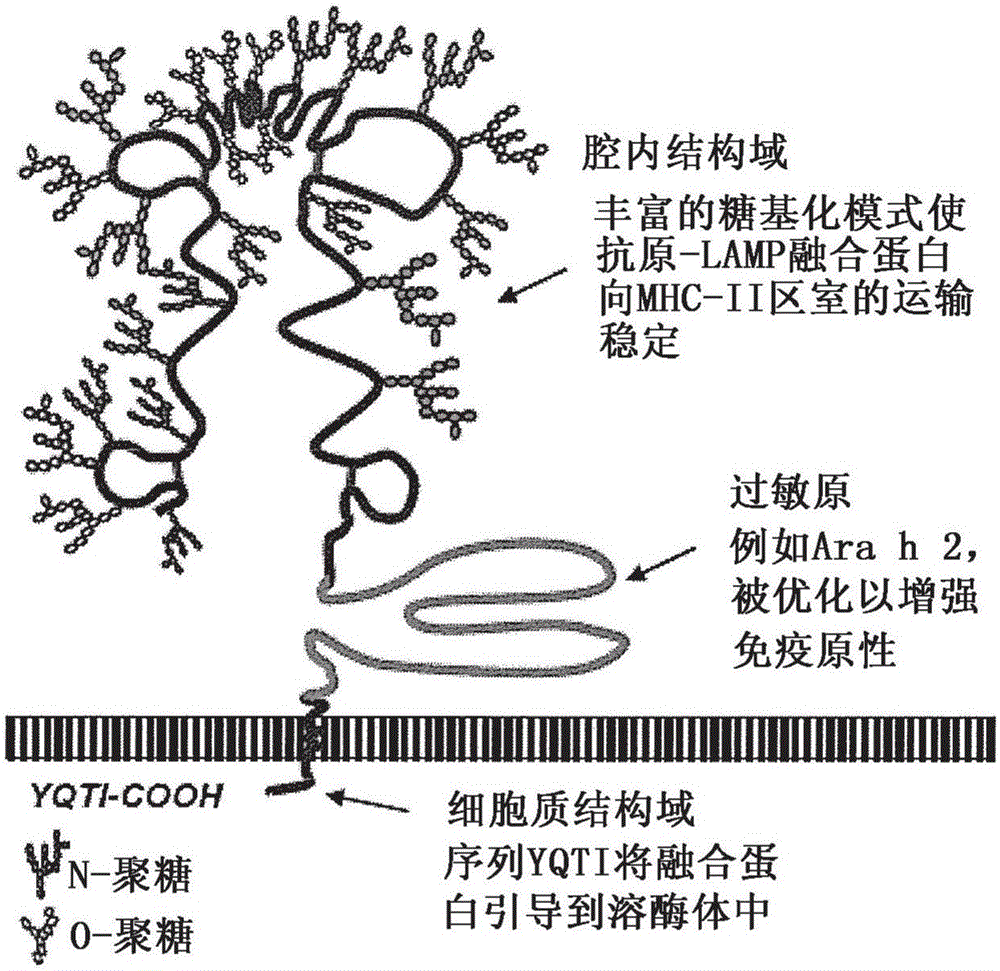
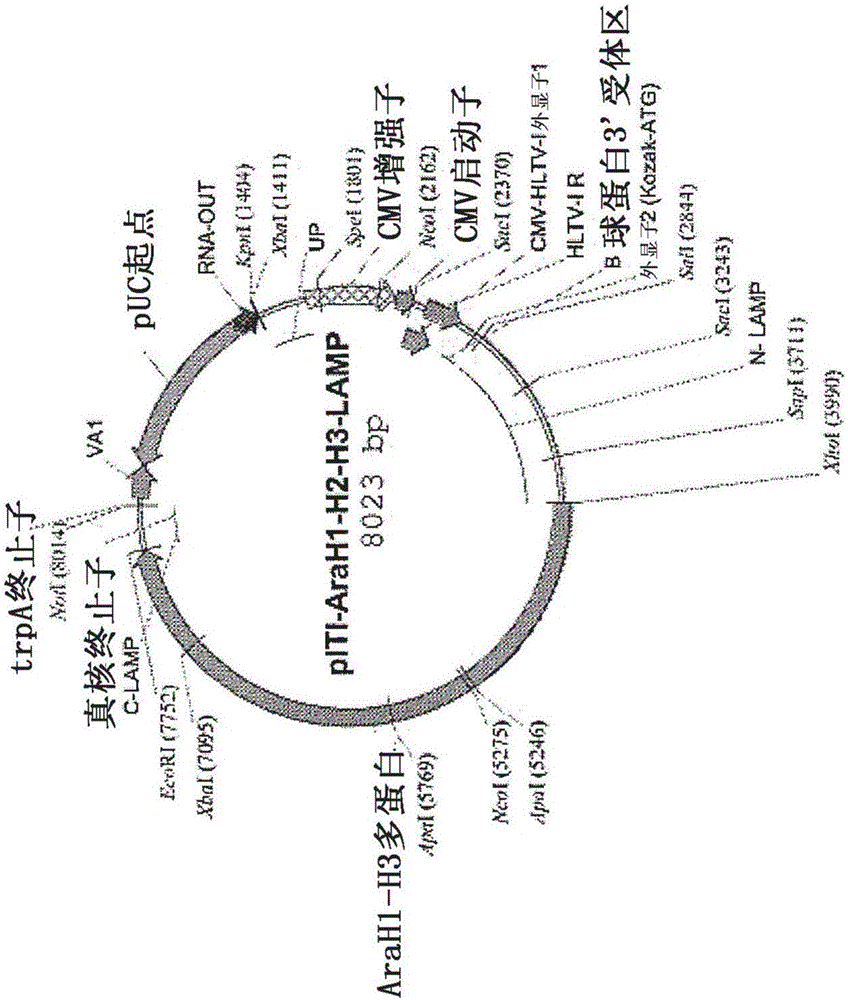
Provided herein are DNA vaccines for the treatment of peanut allergies. The vaccines comprise the coding sequence for one or more peanut allergenic epitopes fused in-frame with the luminal domain of the lysosomal associated membrane protein (LAMP) and the targeting sequence of LAMP. The vaccines can be multivalent molecules and / or can be provided as part of a multivalent vaccine comprising two or more DNA constructs.
Owner:IMMUNOMIC THERAPEUTICS
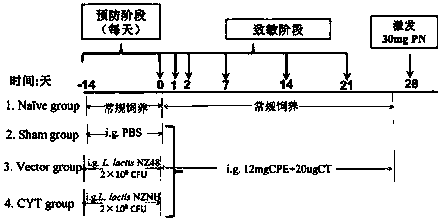
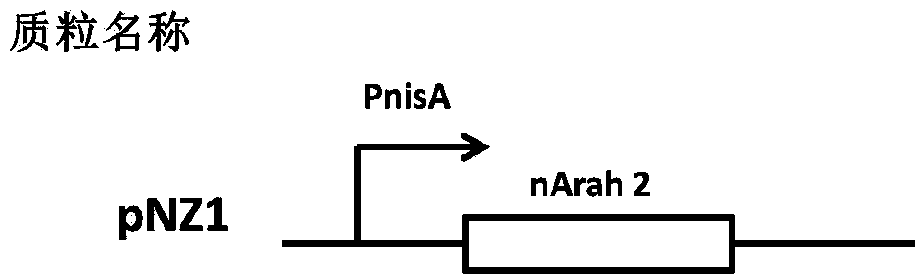
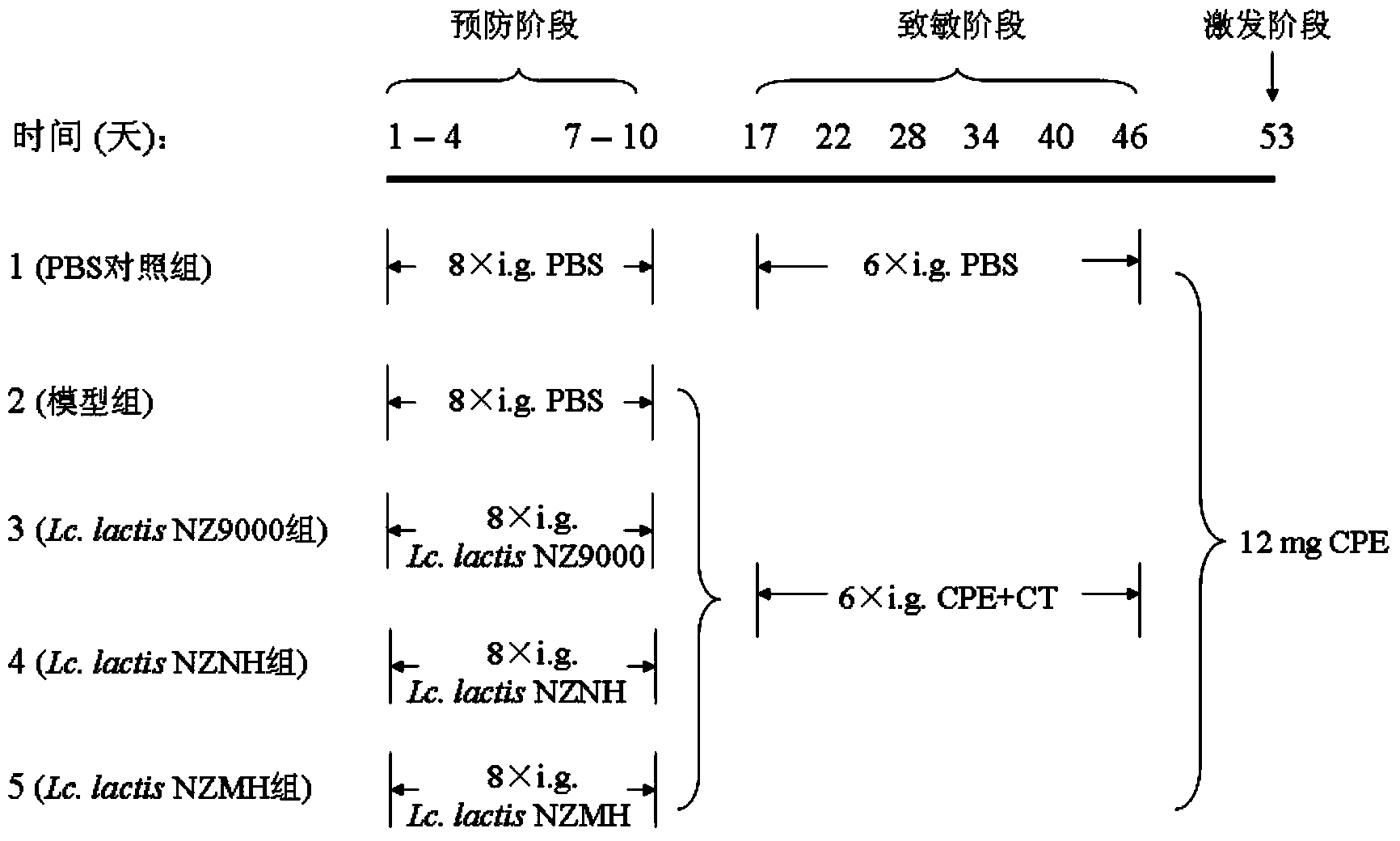
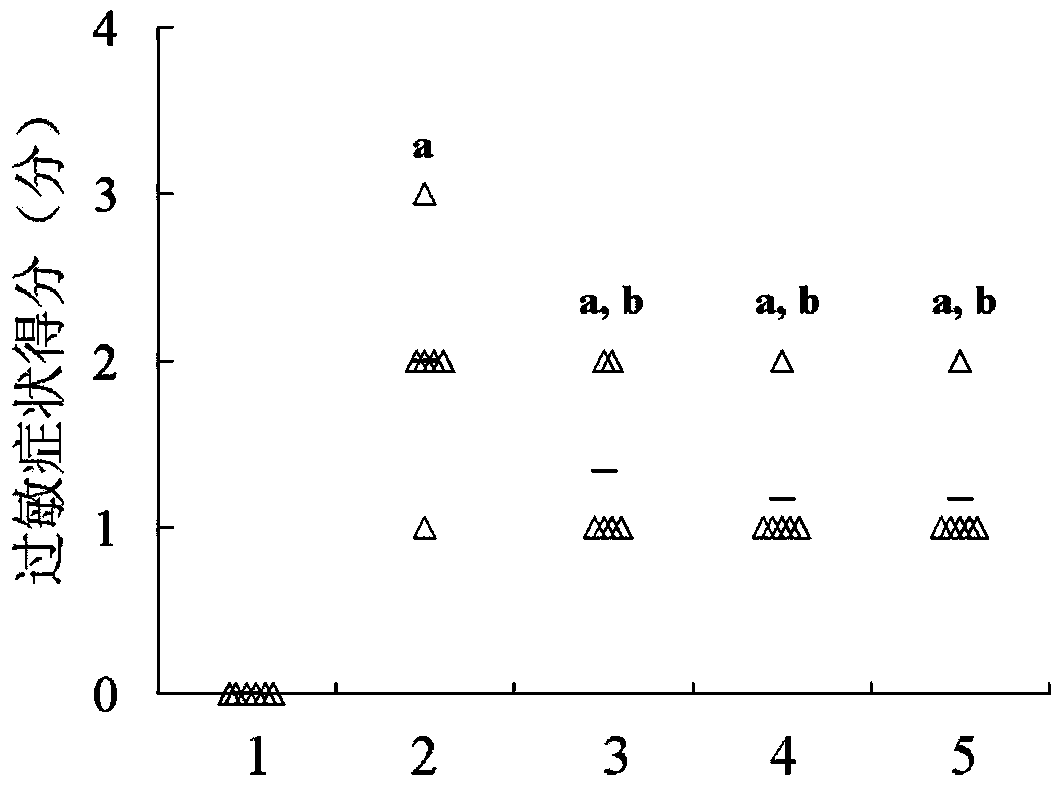
Recombinant lactococcus lactis for intracellular expression of peanut allergen and application thereof
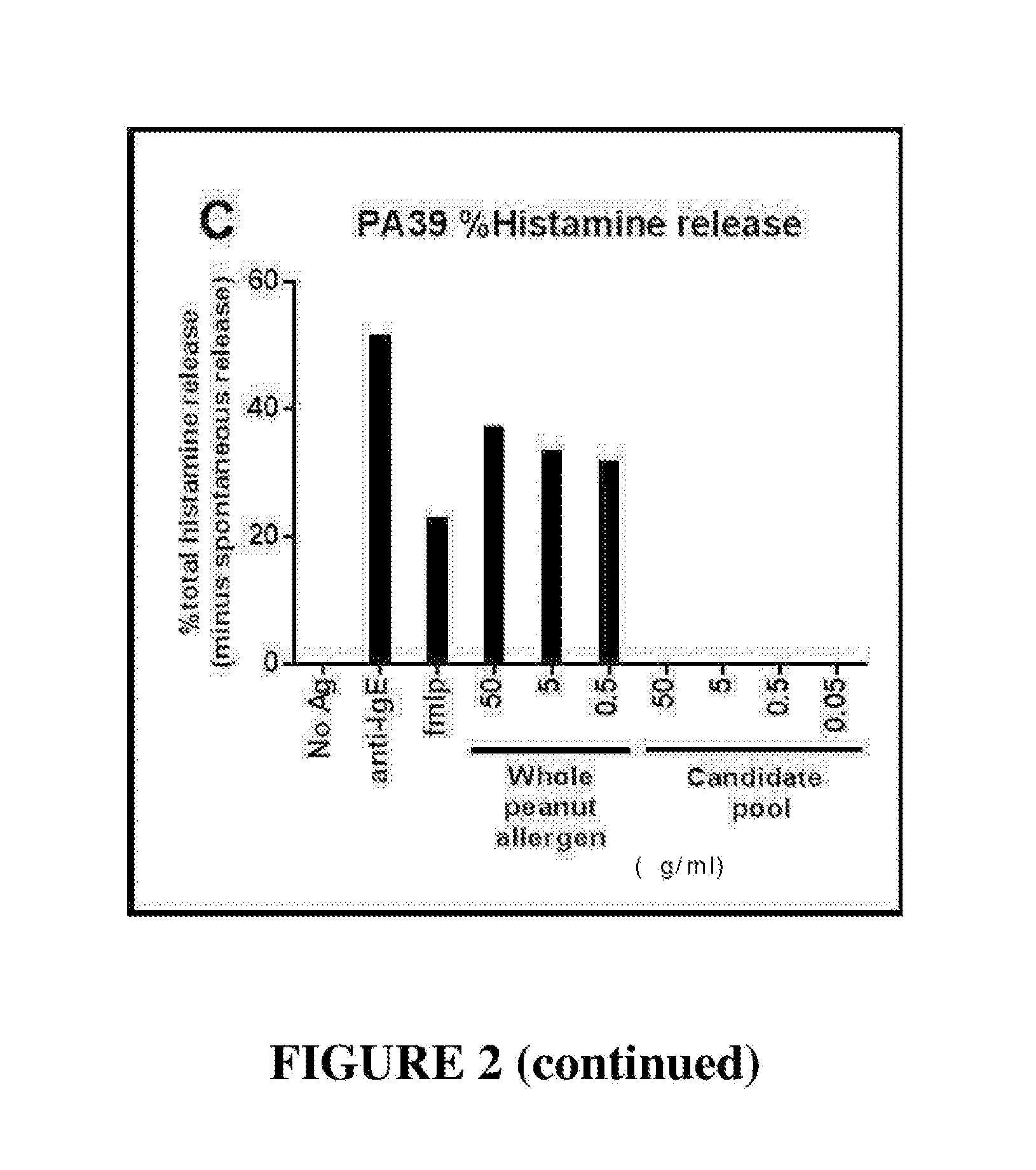
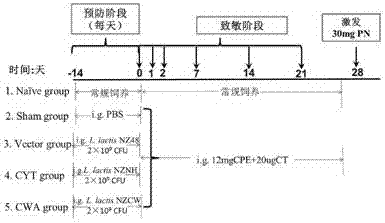
The invention discloses a building method and application of a recombinant lactococcus lactis for intracellular expression of a peanut allergen Arah2. A natural gene sequence of the peanut allergen Arah2 is subjected to codon optimization, so as to obtain an optimized gene nArah2, and a recombinant vector and lactococcus lactis engineering bacteria aiming at an intracellular expression mode of the nArah2 are built. A recombination strain disclosed by the invention is taken as an oral vaccine to immunize the mice, and the immunoreaction of mice anaphylaxis can be effectively regulated. Therefore, the recombinant lactococcus lactis can be developed as a mucosal vaccine to be applied to prevention and treatment of peanut allergy.
Owner:JIANGNAN UNIV
Novel immunotherapeutic composition and uses thereof
ActiveUS20160375130A1Reduce functionReduce presenceOrganic active ingredientsPeptide/protein ingredientsPeanut food allergyProphylactic treatment
The present invention relates generally to an immunotherapeutic composition. More particularly, the present invention relates to an immunotherapeutic composition which interacts immunologically with T lymphocytes in subjects having peanut allergy or allergy to other tree nuts. This composition is preferably immunointeractive with T cells in subjects having an allergy to the Ara h 1 and / or Ara h 2 allergens. The composition of the present invention is useful in the therapeutic or prophylactic treatment of conditions characterised by an aberrant, inappropriate or otherwise unwanted immune response to peanut, Ara h 1 and / or Ara h 2 or derivative or homologue thereof.
Owner:ARAVAX
Preparation method and application of recombinant lactococcus lactis capable of displaying peanut allergen on surface
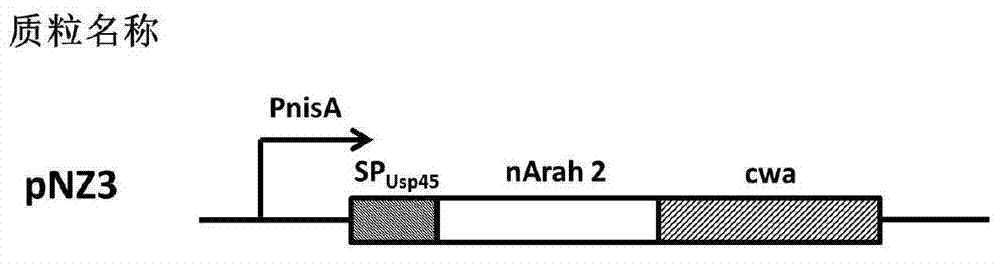
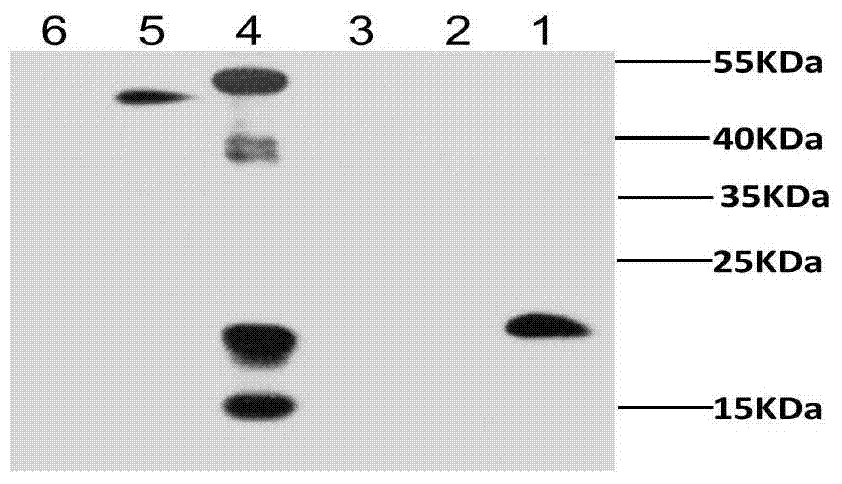
The invention discloses a construction method and an application of recombinant lactococcus lactis capable of displaying a peanut allergen Arah2 on the surface. An optimized gene nArah2 is obtained by performing codon optimization on a natural gene sequence of the peanut allergen Arah2, and a recombinant vector for a cell wall-anchored expression way of the nArah2 and engineering lactococcus lactis are constructed. A related recombinant strain is used as an oral vaccine for mouse immunization, and the allergic immune response of mice can be effectively adjusted. Therefore, the recombinant strain can be developed into a mucosal vaccine for the prevention and control of peanut allergy.
Owner:JIANGNAN UNIV
Treatment for peanut allergy
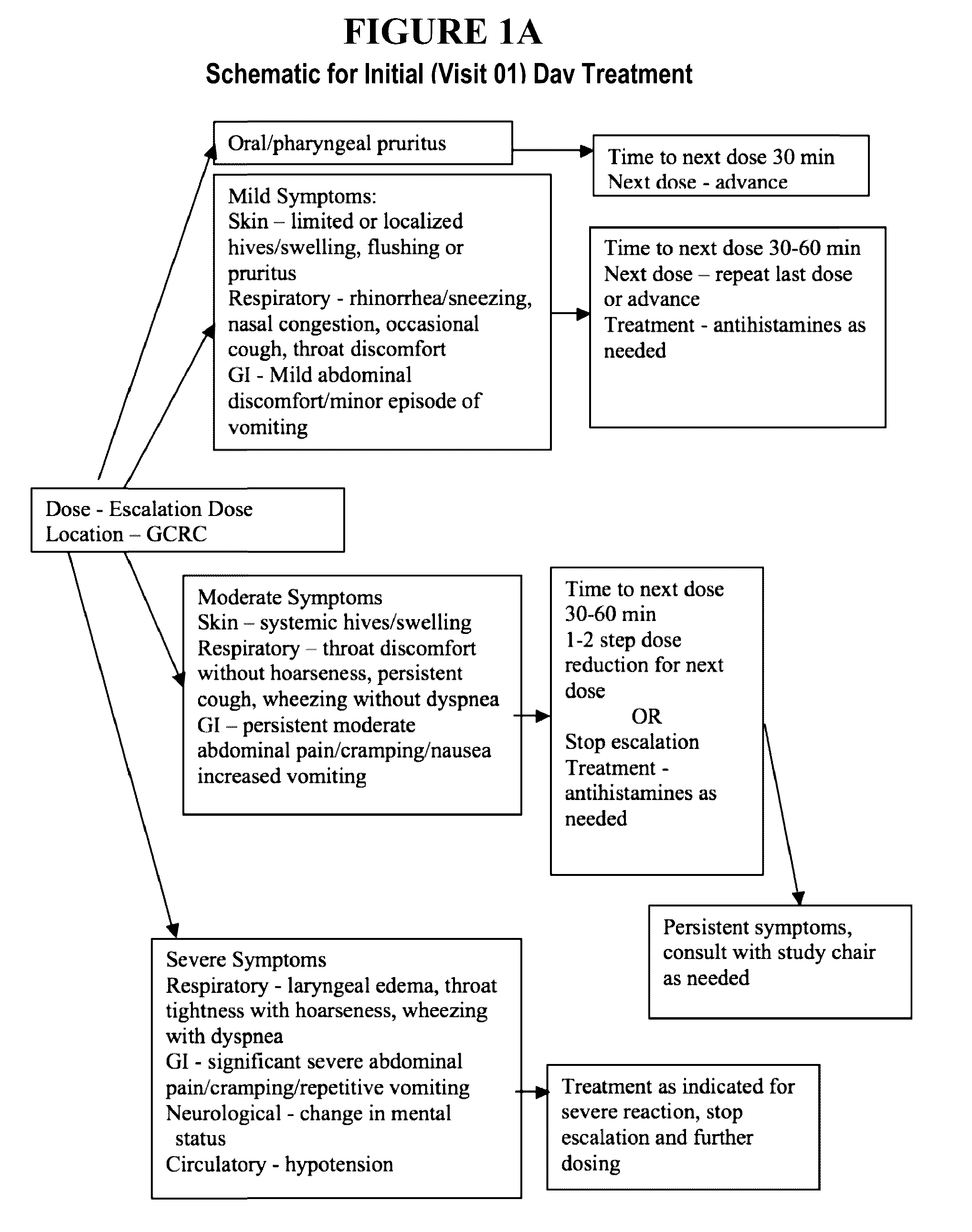
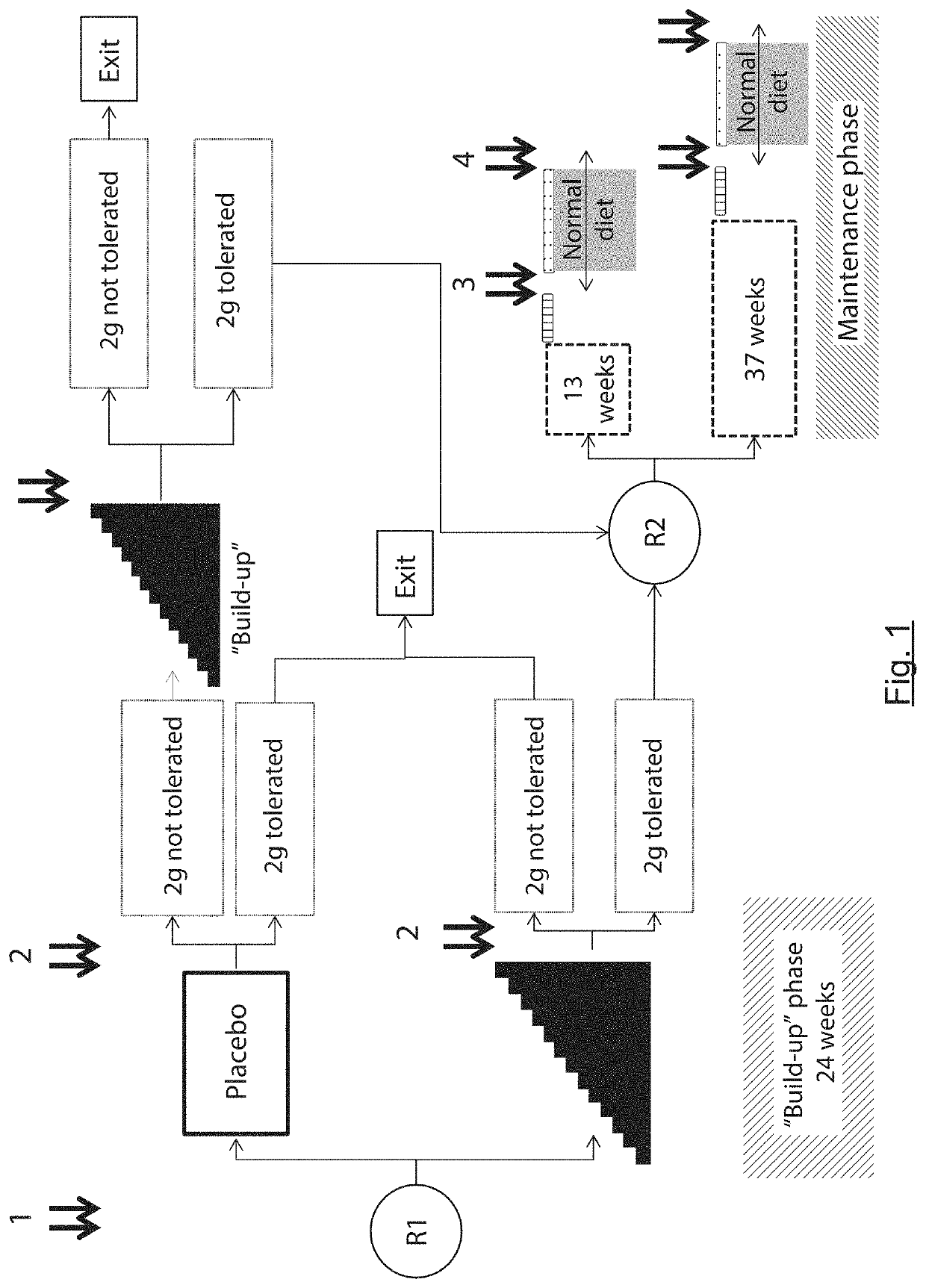
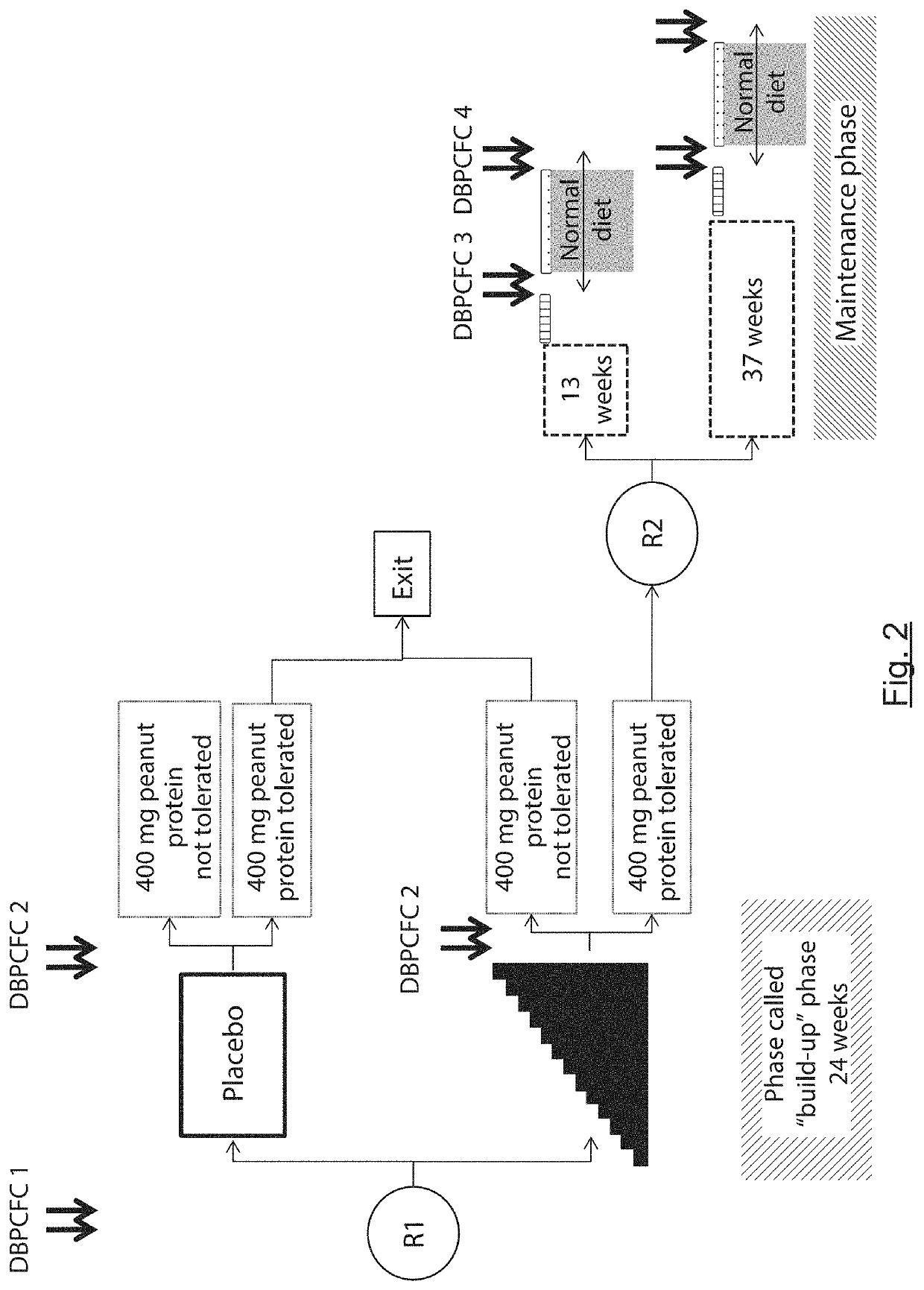
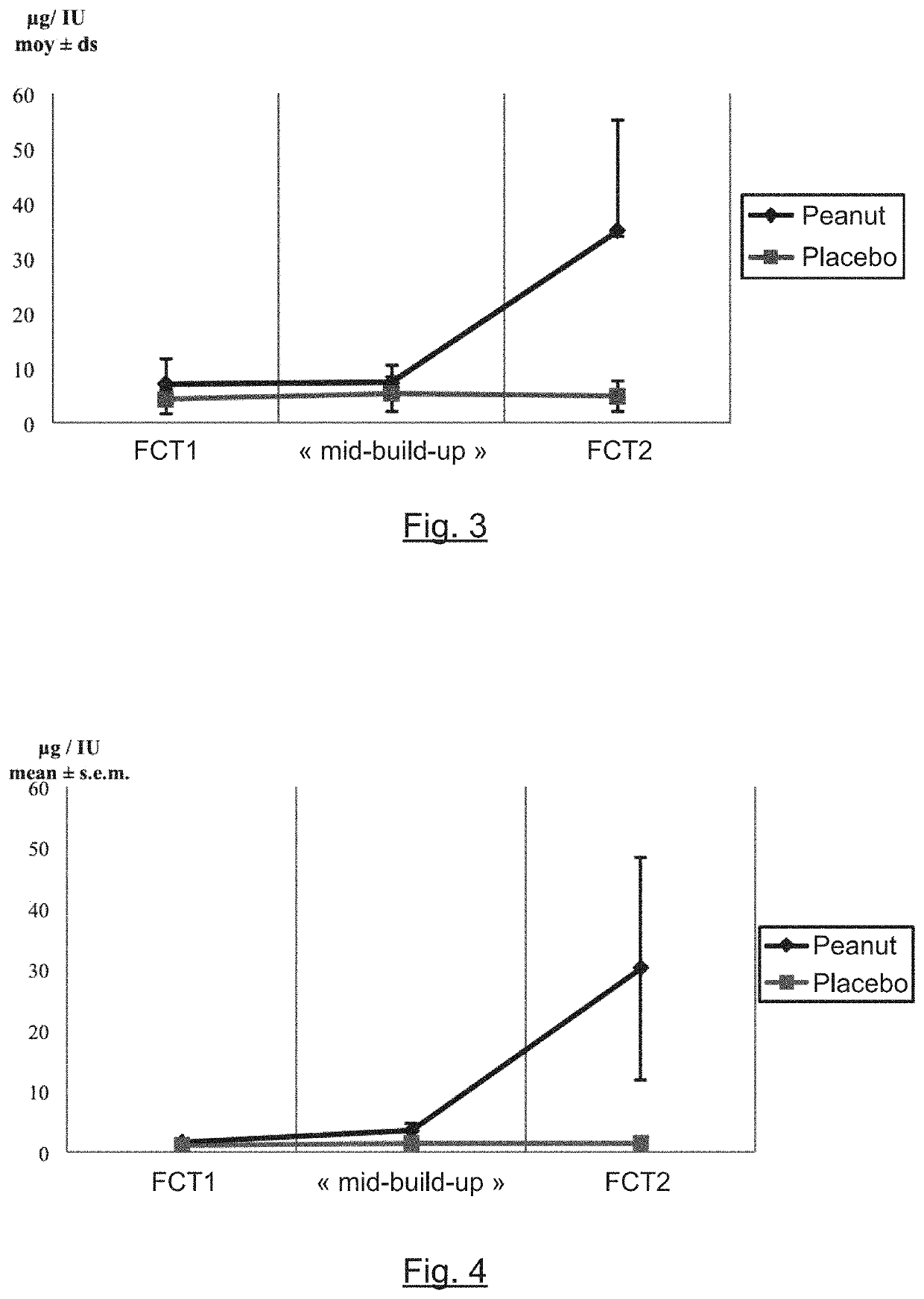
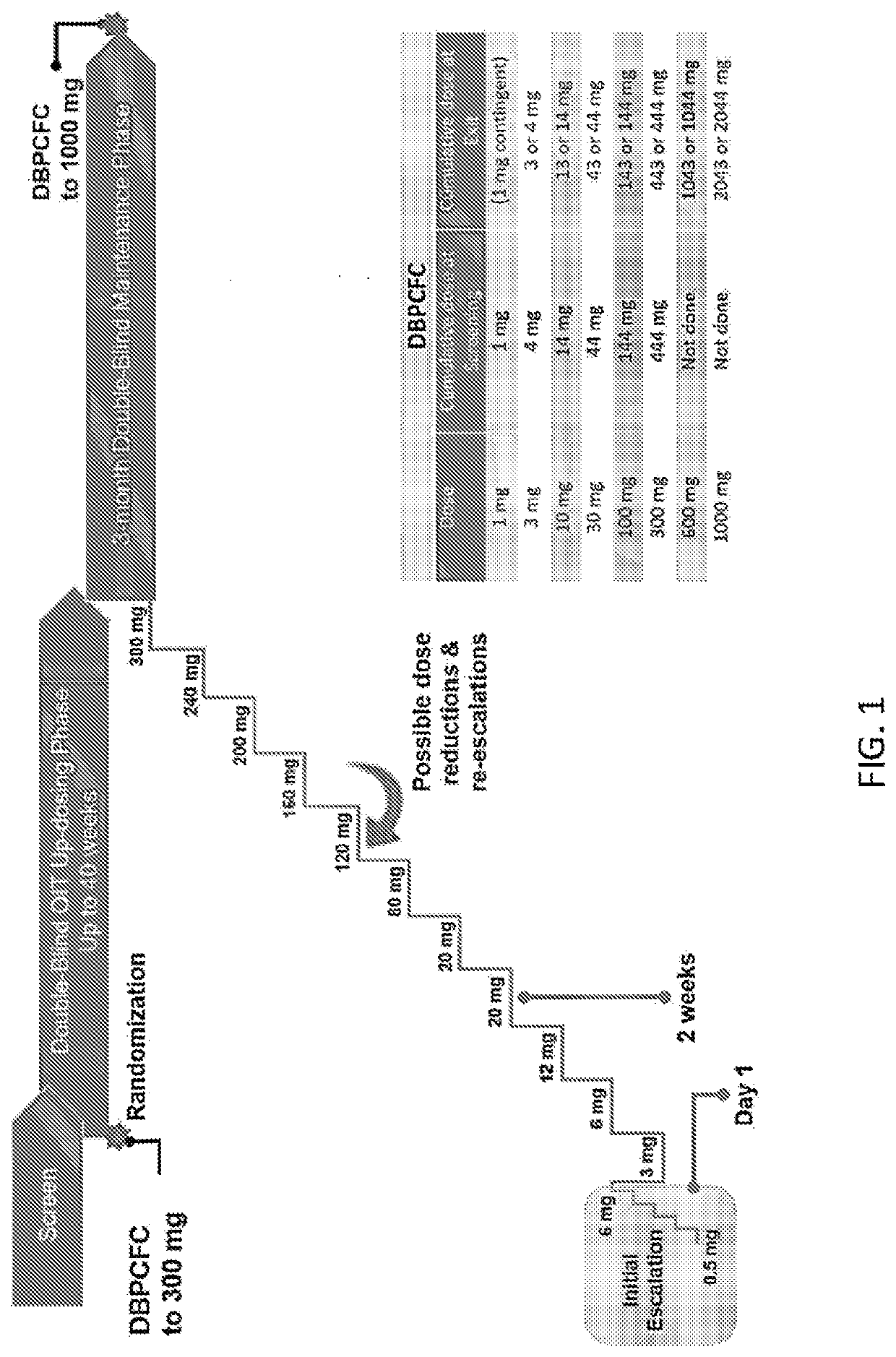
The present invention relates to oral immunotherapy for the desensitisation of patients who are hypersensitive to peanut allergen. The immunotherapy comprises increasing the oral dose daily dose of peanut protein administered to the patient at intervals of at least 2 weeks in a series of increments from an initial dose to a maximum dose, the series of dose increments including 2mg, 5mg, 12.5mg, 25mg, 50mg, 0mg, 200mg, 400mg and 800mg; administering a daily oral dose of the maximum dose of peanut protein for at least 2 years and then administering a weekly oral dose of the maximum dose of peanut protein for at least 2 years.
Owner:CAMBRIDGE ENTERPRISE LTD +1
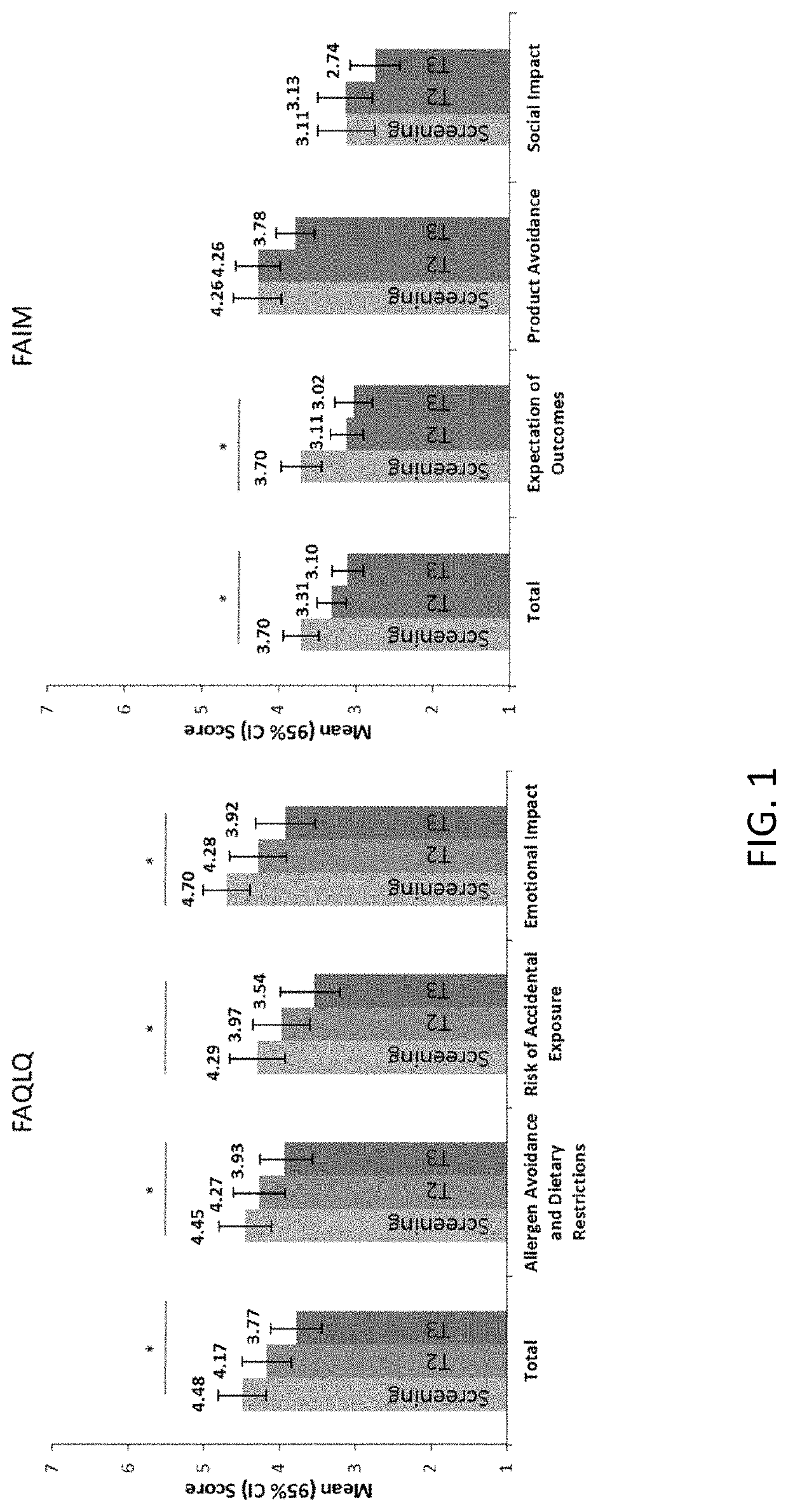
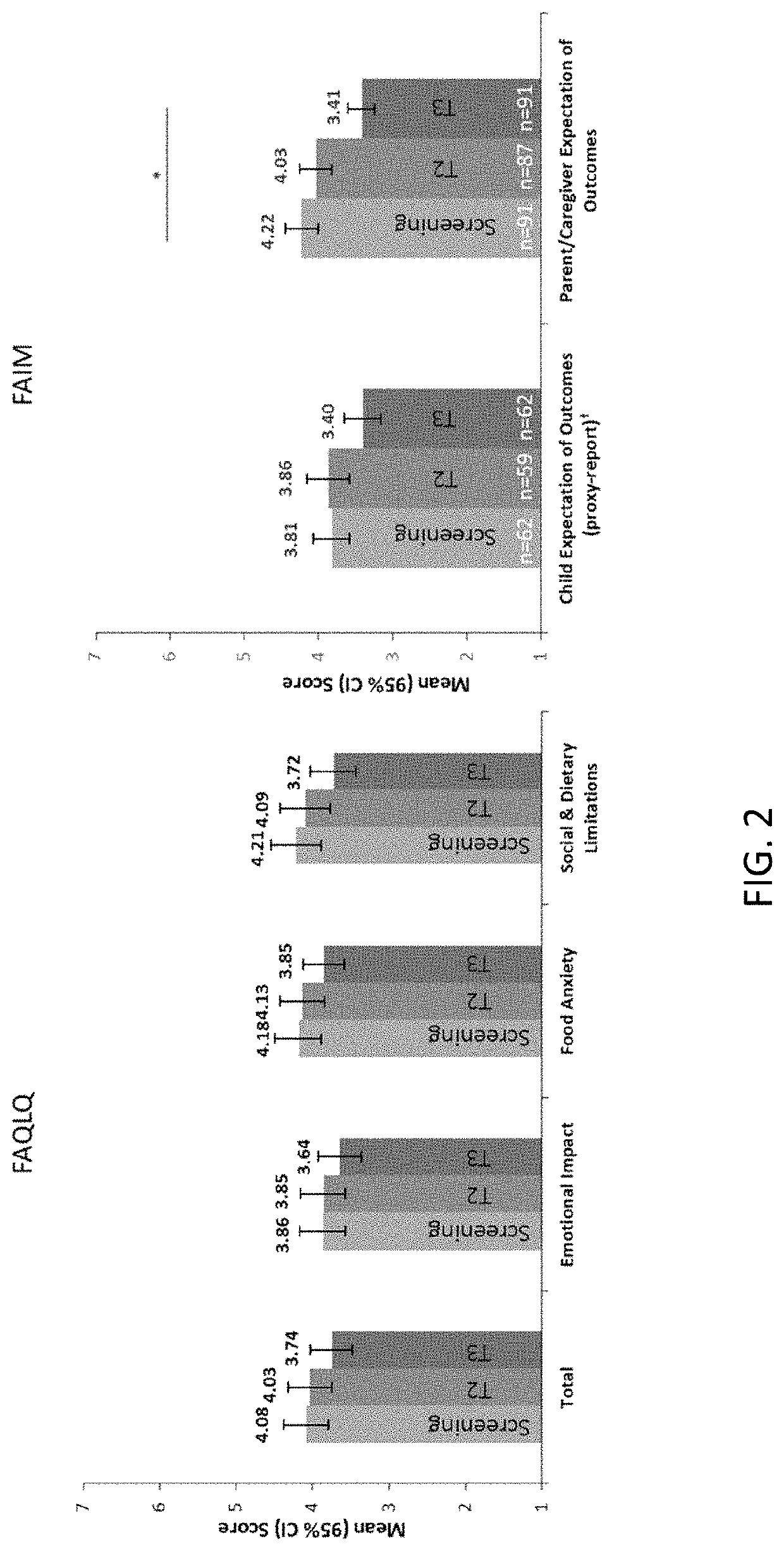
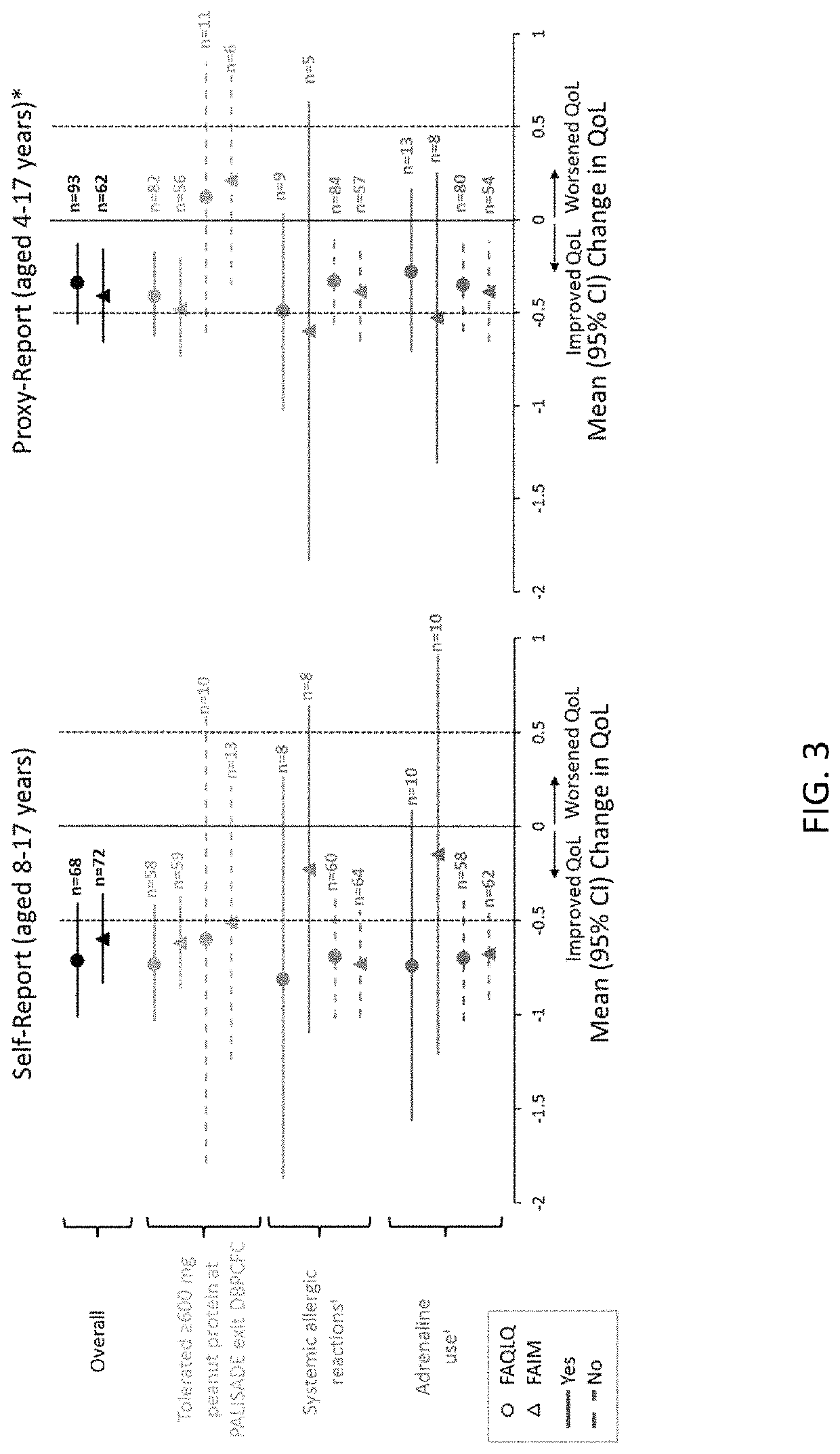
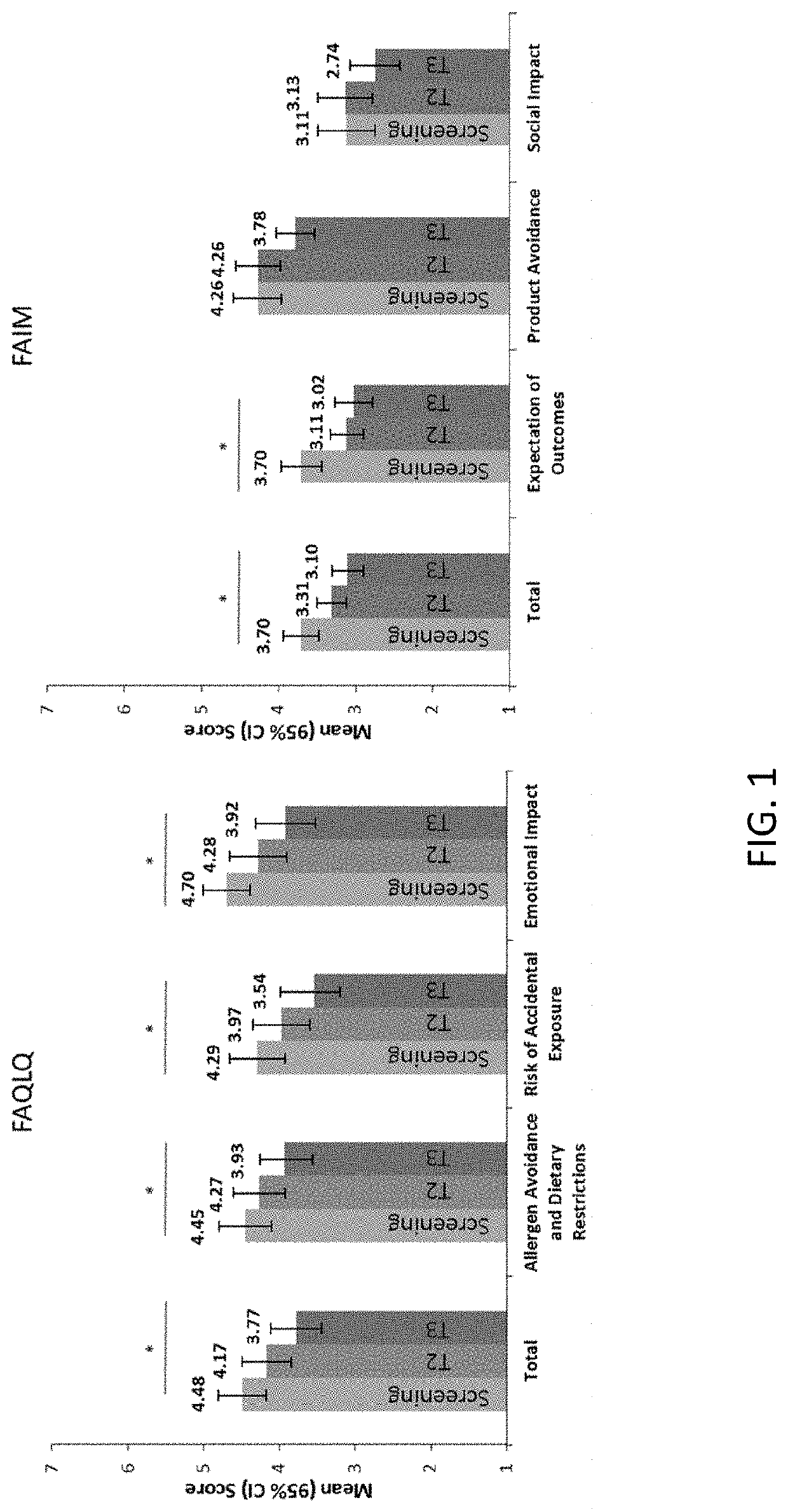
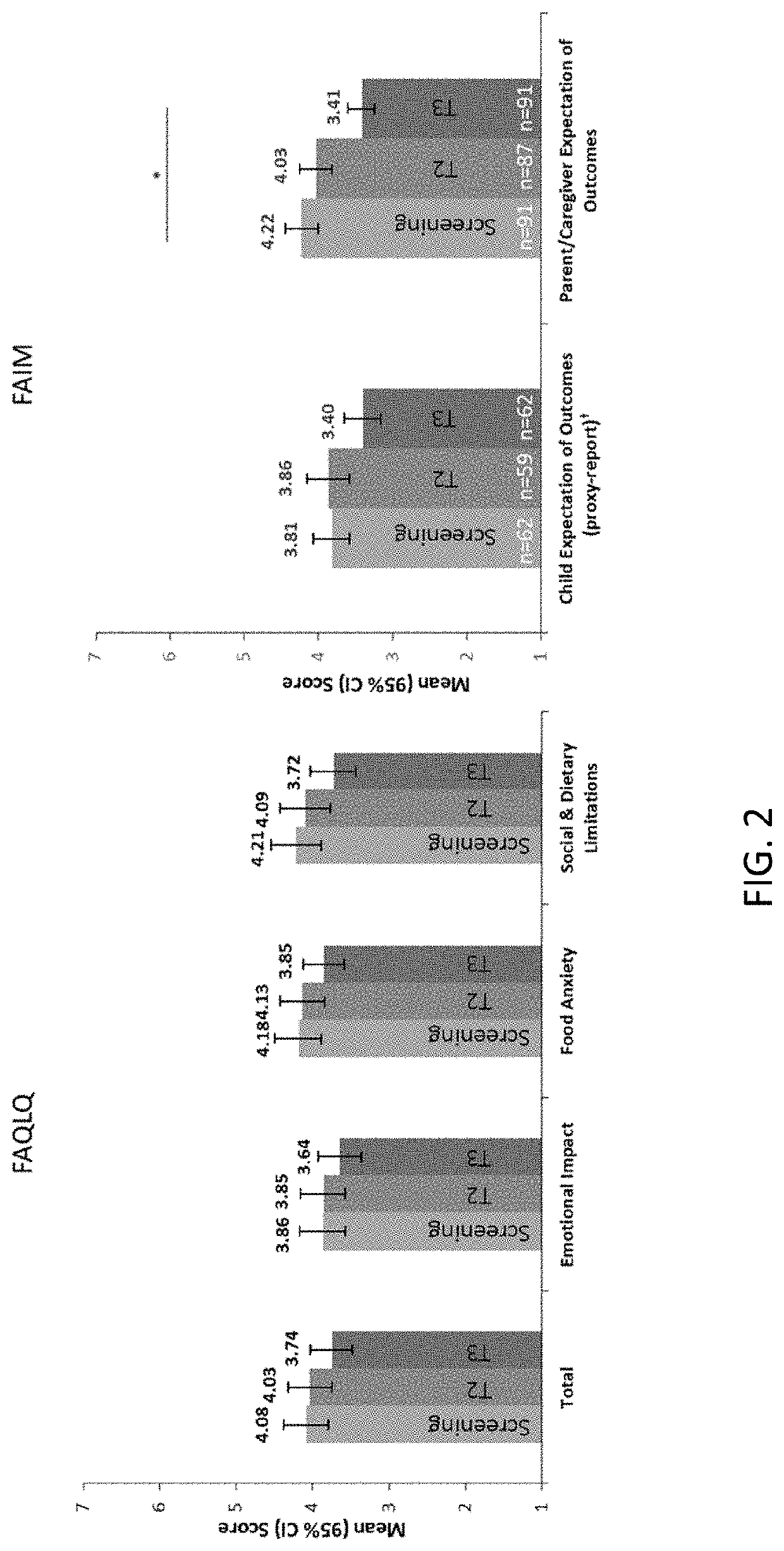
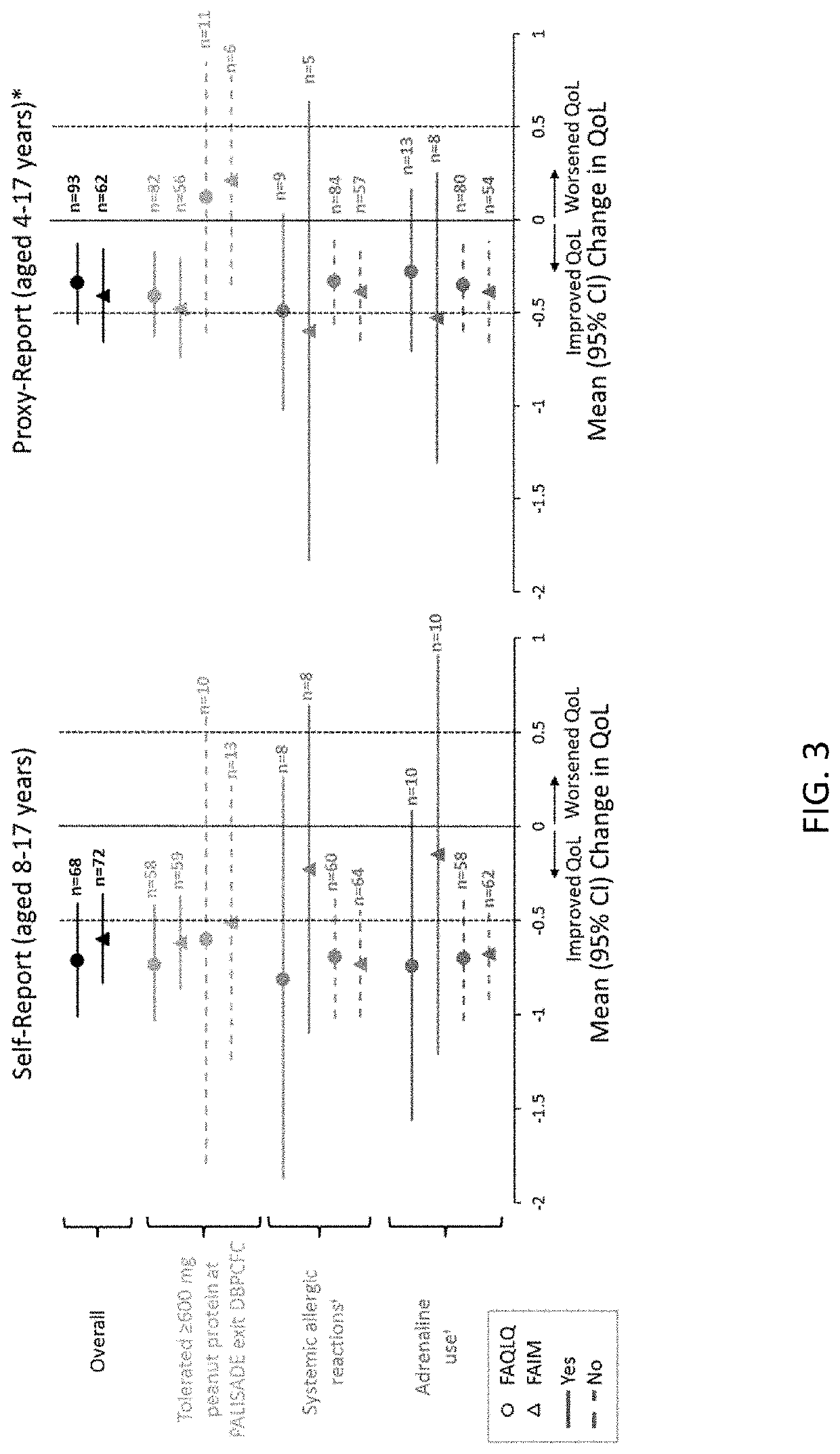
Methods for improving the quality of life of a patient with a peanut allergy
ActiveUS11229673B2Improve the quality of lifePharmaceutical delivery mechanismImmunological disordersPeanut food allergyOral immunotherapy
The present disclosure relates to methods of improving the quality of life of a subject having a peanut allergy. In certain embodiments, the disclosure provides methods for improving the quality of life of a subject having a peanut allergy by administering a peanut composition according to an oral immunotherapy schedule. In certain embodiments, the disclosure provides methods for improving the quality of life of a subject having a peanut allergy by informing the subject they are to be, or are being, administered a peanut composition according to an oral immunotherapy schedule.
Owner:SOC DES PROD NESTLE SA
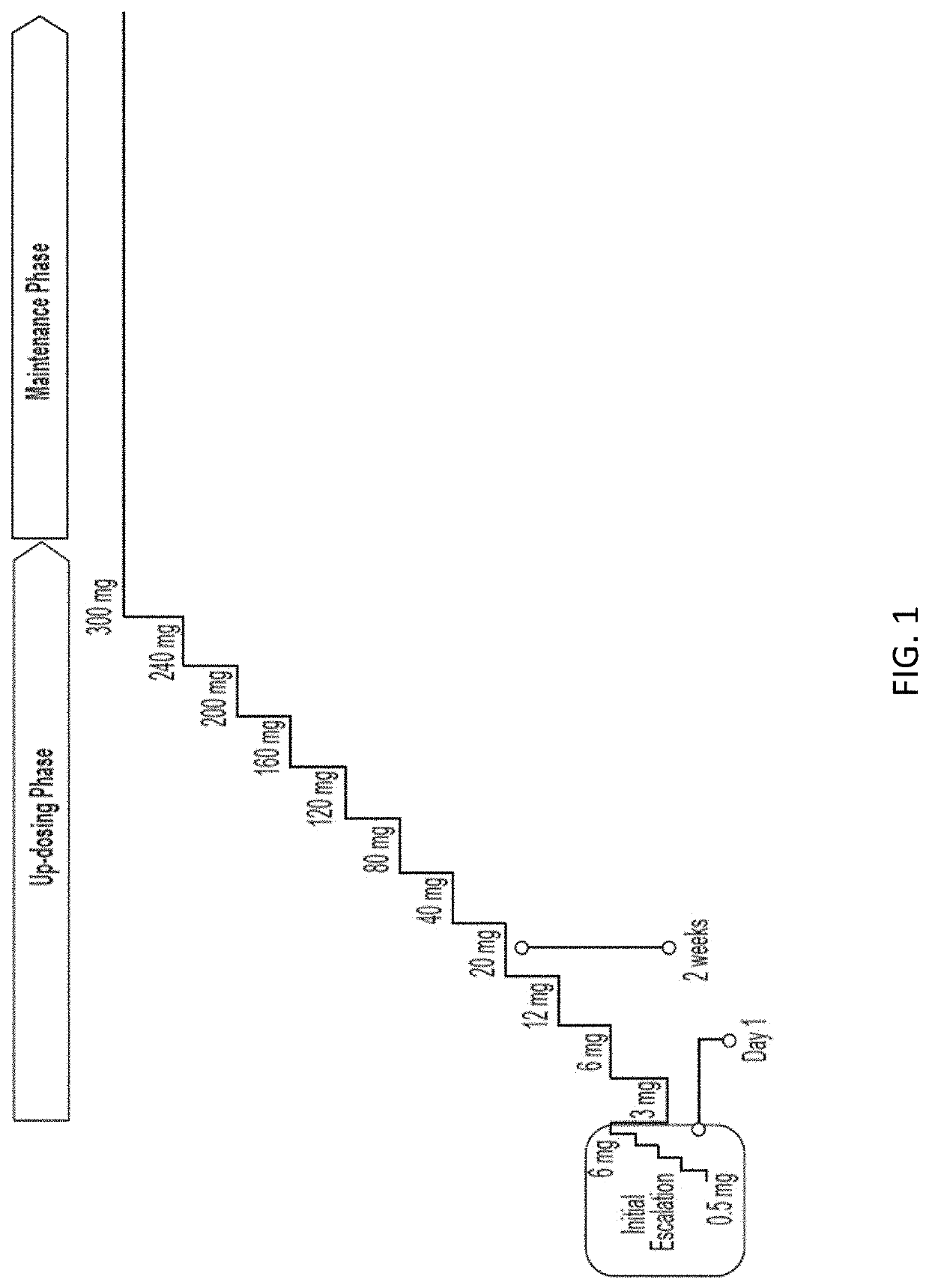
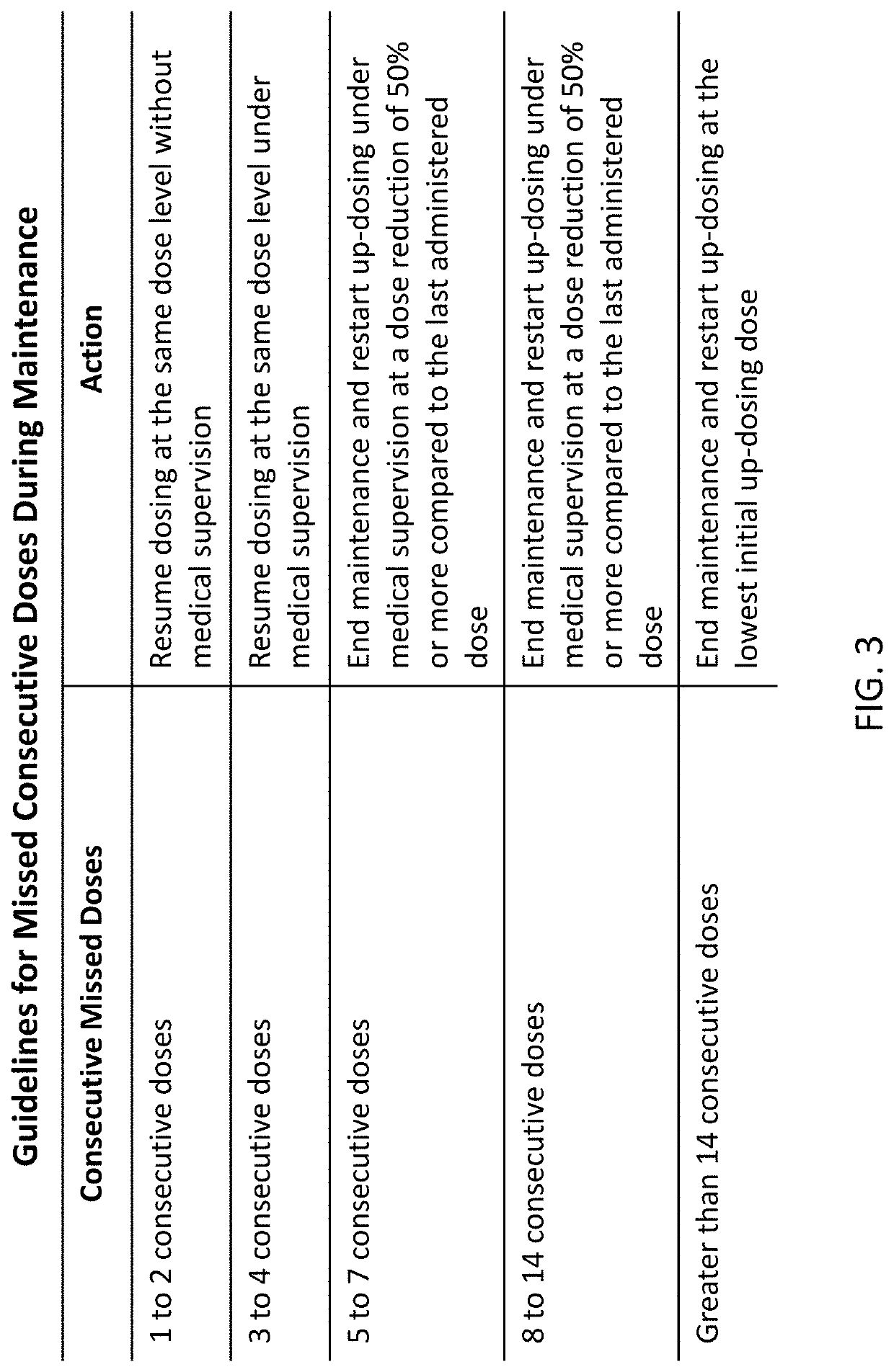
Peanut oral immunotherapy with maintenance dose
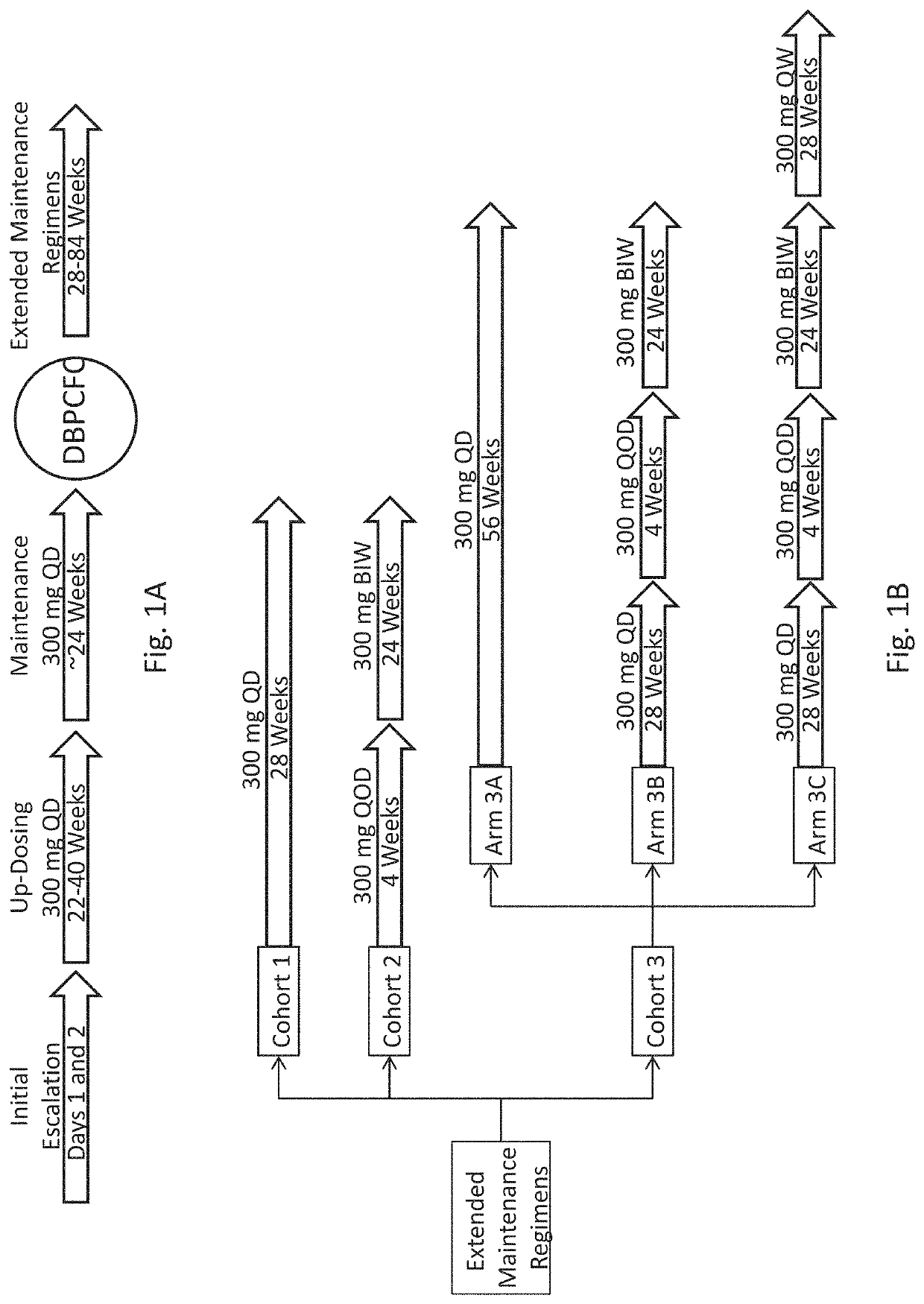
PendingUS20200054738A1Increasing frequency of administrationHigh frequencyPharmaceutical delivery mechanismAllergen ingredientsPeanut food allergyOral immunotherapy
The present disclosure relates to improved oral immunotherapy methods for treating peanut allergy. The disclosure provides methods for treating a subject for a peanut allergy by an oral immunotherapy comprising an up-dosing phase and a maintenance phase. In certain embodiments, the methods comprise non-daily administration to the subject of a maintenance phase dose. In certain embodiments, the methods comprise administering a maintenance phase dose for more than 24 weeks. In other embodiments, the disclosure provides methods for reducing the risk of an allergic adverse event in a subject receiving an oral immunotherapy dosage therapy.
Owner:SOC DES PROD NESTLE SA
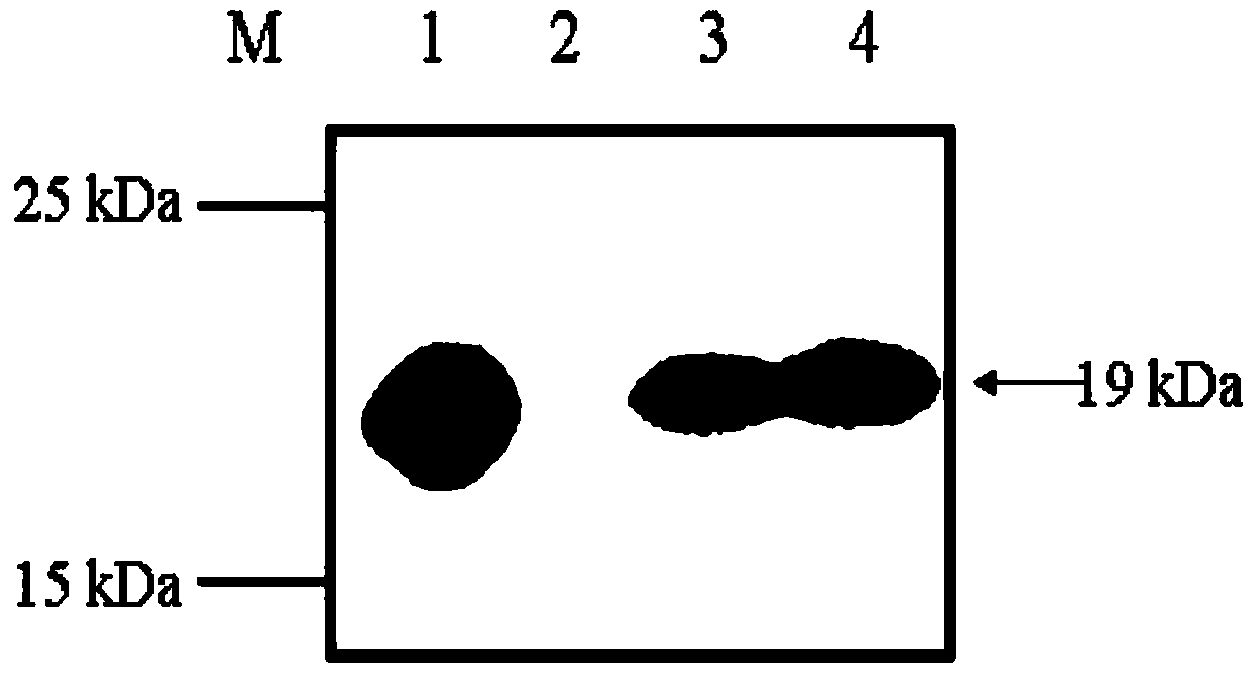
Lactococcus lactis recombinant bacteria for expressing hypoallergenic peanut allergen, and application thereof
InactiveCN103626860AAllergenicity reductionLittle side effectsBacteriaMicroorganism based processesBiotechnologyPeanut food allergy
The invention discloses lactococcus lactis recombinant bacteria for expressing hypoallergenic peanut allergen, and application thereof. According to the invention, on the basis of retaining natural peanut allergen Arah2 protein immunogenicity, the allergenicity is lowered, codon optimization and modified mutation are performed on Arah2 so as to obtain an optimized gene mArah2, and recombinant expression vectors and recombinant lactococcus lactis strains aiming at mArah2 are built. By using the recombinant strains in the invention for rat immunization, peanut allergy can be effectively restrained, and the immune protection effect is remarkable. Therefore, the lactococcus lactis recombinant bacteria for expressing hypoallergenic peanut allergen have a great potential on the aspect of curing peanut allergy.
Owner:JIANGNAN UNIV
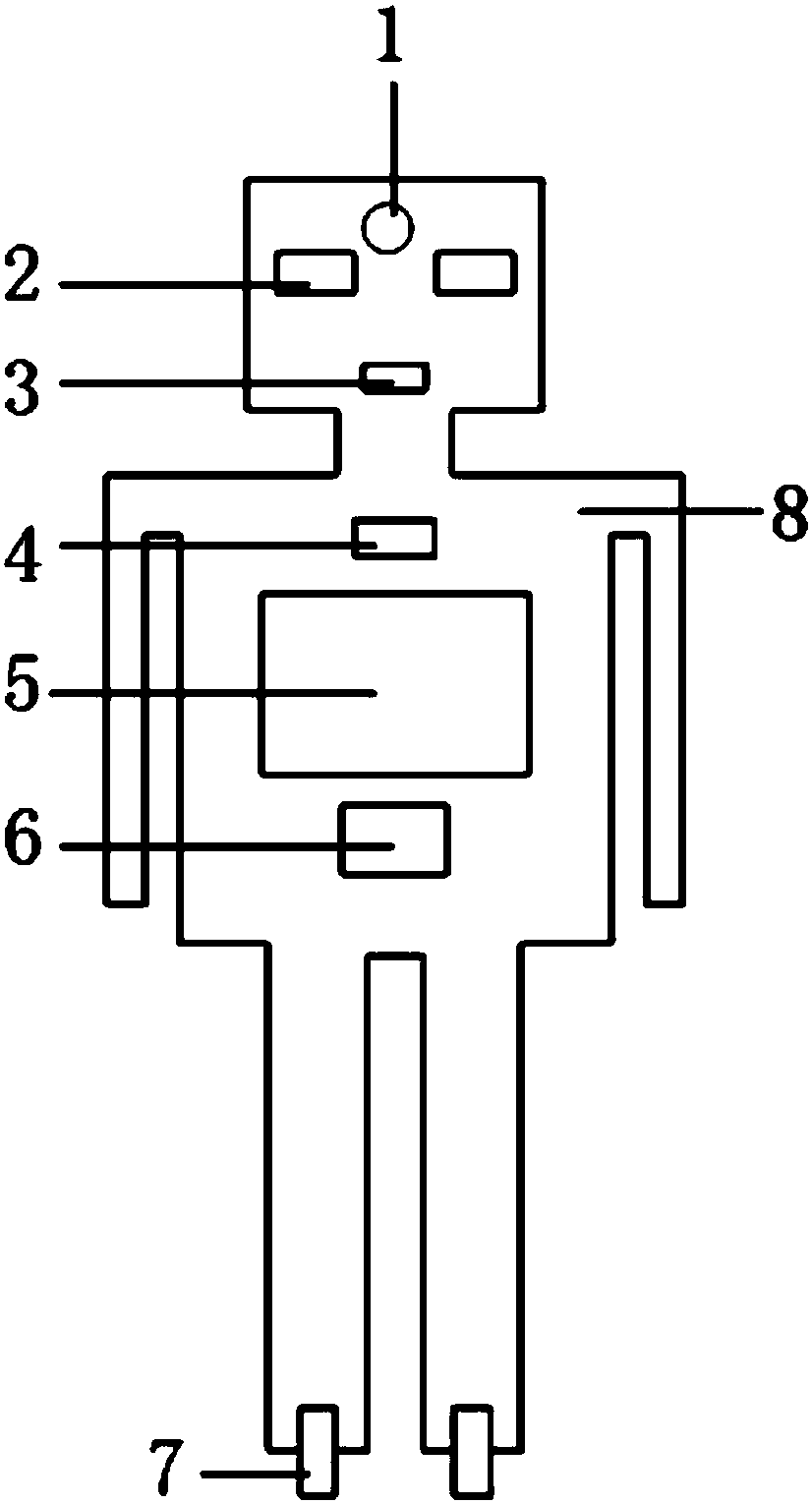
Kitchen robot used for guiding cooking
The invention relates to a kitchen robot used for guiding cooking. The kitchen robot comprises a camera (1), face ornaments (2), a taste sensor (3), an on-off key (4), a display screen (5), a soundingdevice (6), wheels (7) and an appearance main body (8). A menu is selected, the diner number or the weight of food materials is input, the flavor is input, and if required, the special requirements (for example, diners suffer from hypertension, are allergic to peanuts and the like) are input together; after data analysis, the analyzed data are transmitted to the display screen (5) through a transmitter, and the display screen displays the specific method; after the materials are prepared, the camera (1) is turned on and made to scan the cooking state of an operator; when the next step is conducted, the sounding device (6) makes a prompting tone, and the next step is conducted according to the prompt tone till cooking is finished; and the taste sensor (3) analyzes and evaluates food cookedby the operator, and the analyzed data are displayed on the display screen. The problem that a recipe and a video are different from actual operation is solved.
Owner:NANJING LEPENG ELECTRONICS TECH CO LTD
Modification of Allergens for Immunotherapy
InactiveUS20150273052A1Alleviate and inhibit any significant allergic reactionEffectively desensitizeAllergen ingredientsImmunological disordersPeanut food allergyImmunotherapy
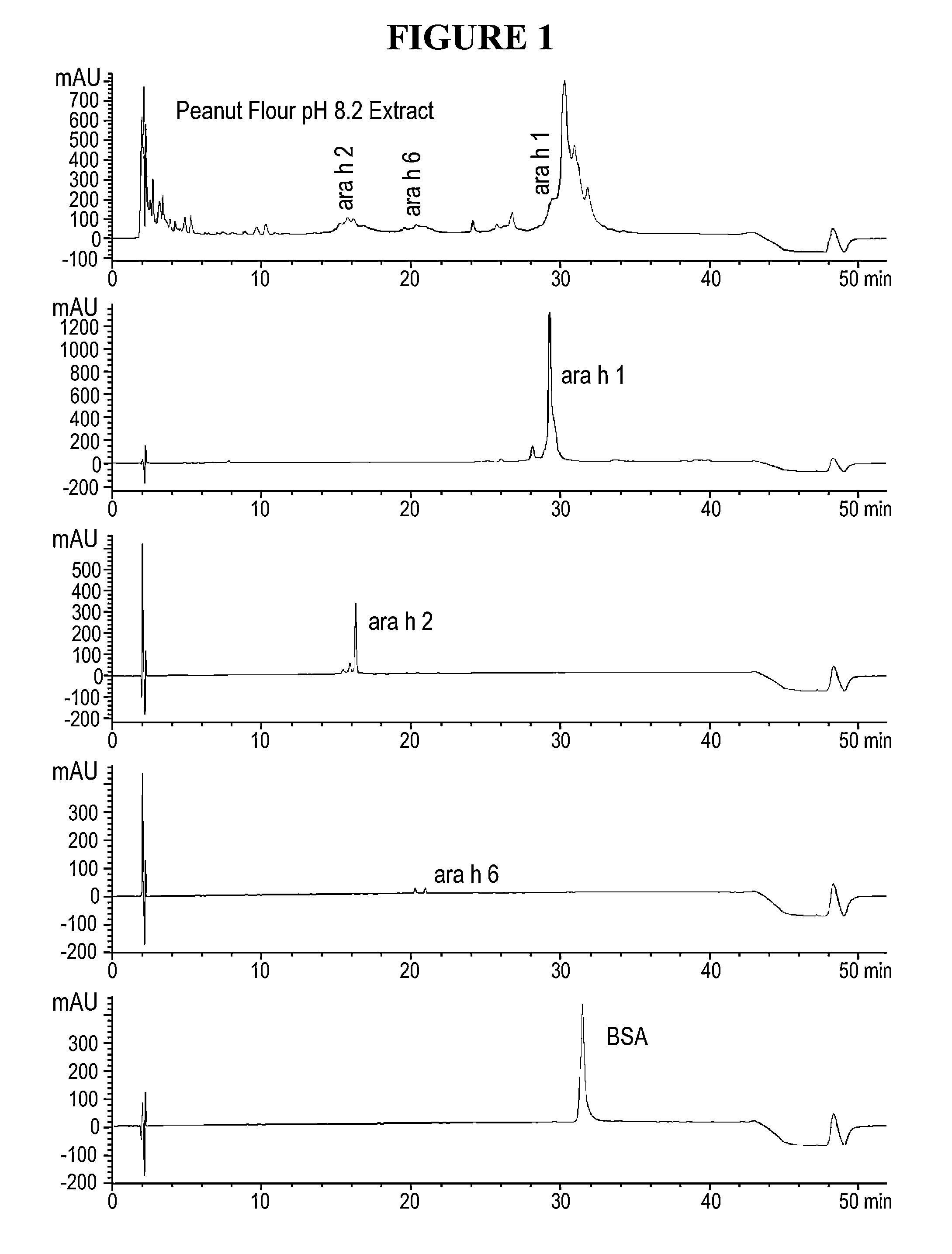
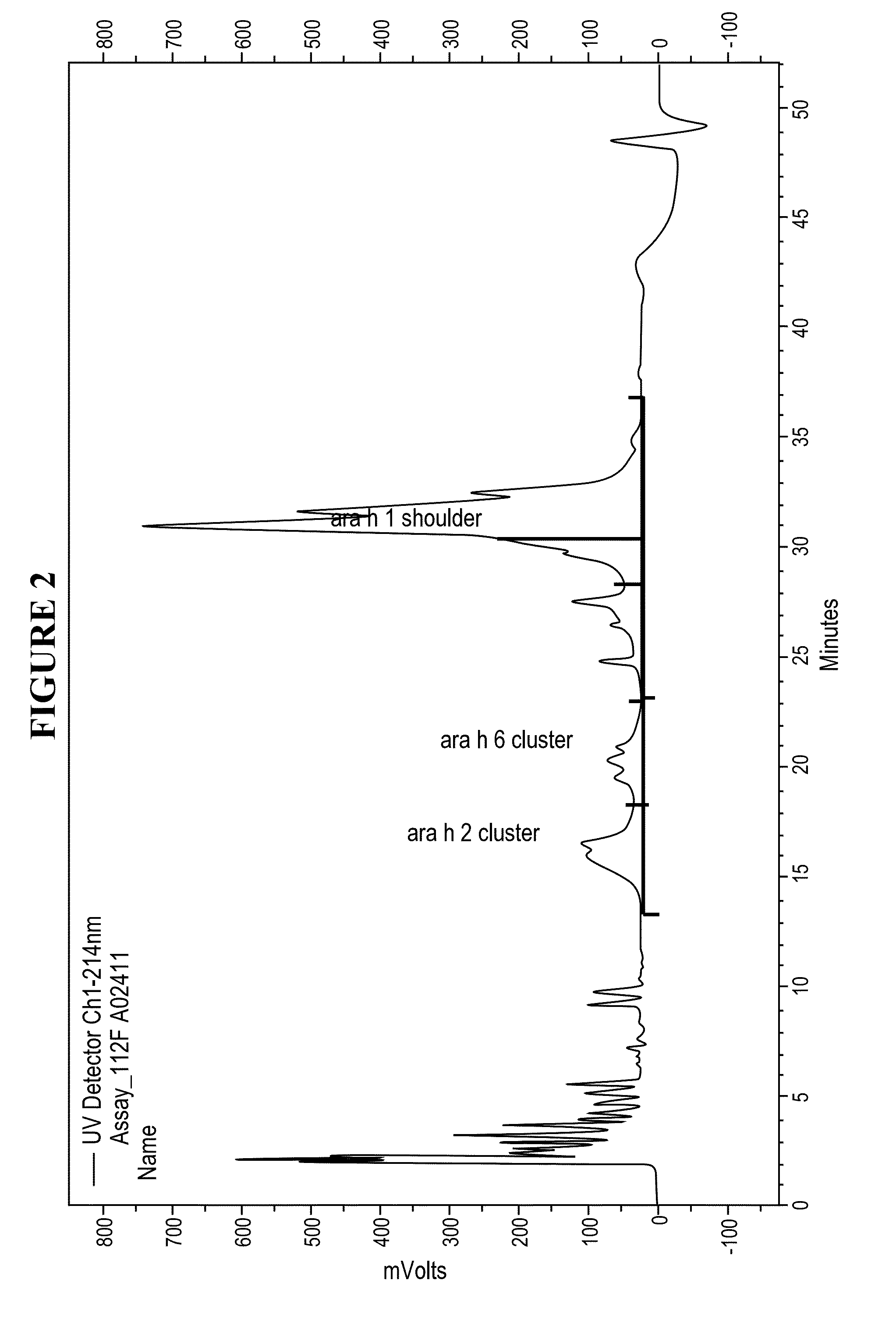
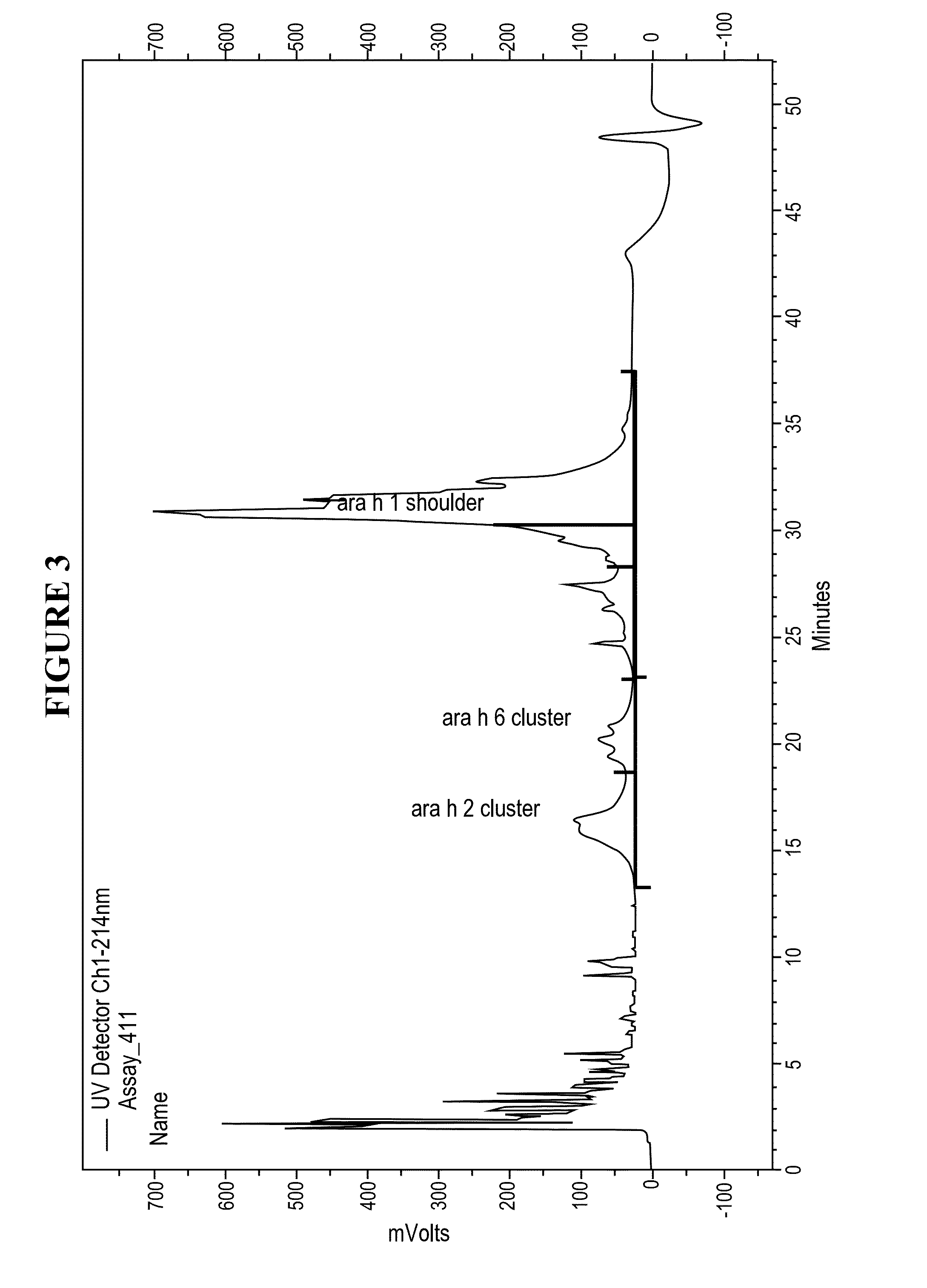
The present invention relates to pharmaceutical compositions for immunotherapy, for example for immunotherapy of peanut allergy. Further, the present invention relates to methods for the preparation of the present pharmaceutical compositions for immunotherapy, and their use in immunotherapy.Furthermore, the present invention relates to processes for modifying allergens thereby enhancing their application in immunotherapy. The invention also relates to the present modified allergens and pharmaceutical compositions comprising the present allergens, as well as to the use thereof in immunotherapy.According to a particularly preferred embodiment, the present invention relates to pharmaceutical compositions for immunotherapy comprising reduced and alkylated naturally occurring peanut allergens Ara h2 and / or Ara h6, or derivates or isoforms thereof wherein said pharmaceutical composition substantially does not comprise Ara h1 and / or Ara h3.
Owner:HAL ALLERGY HLDG
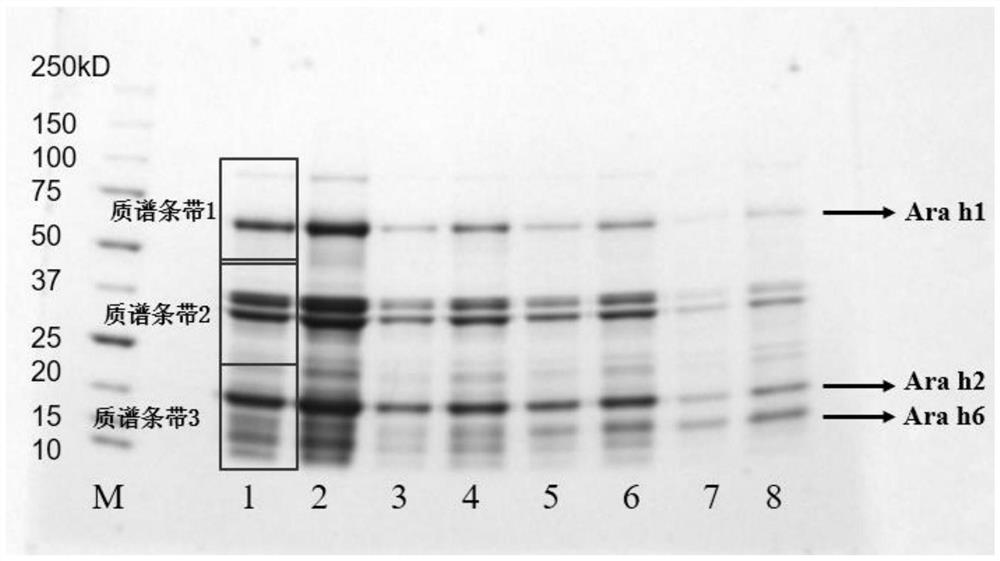
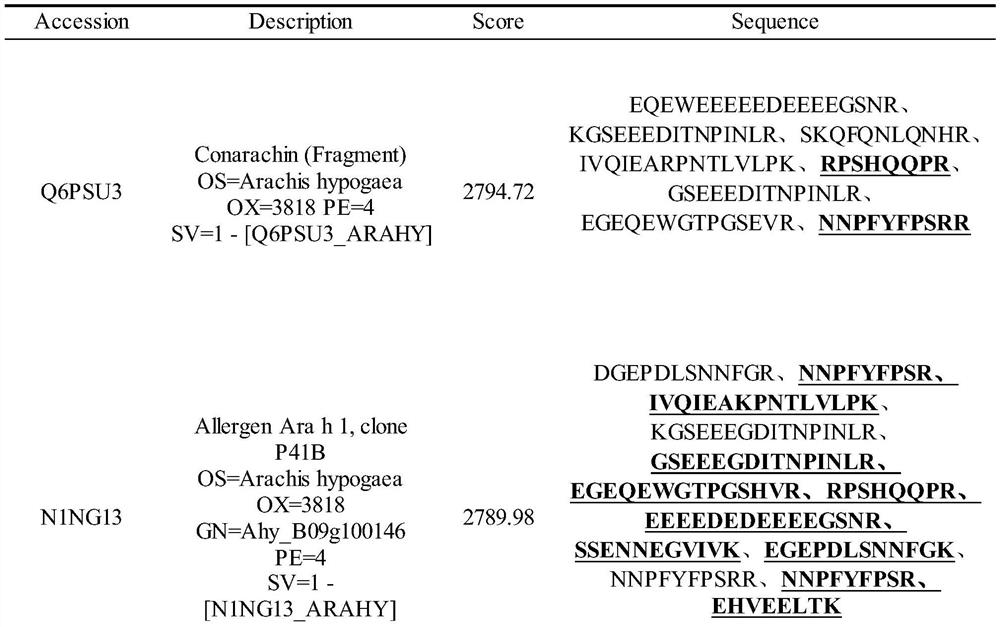
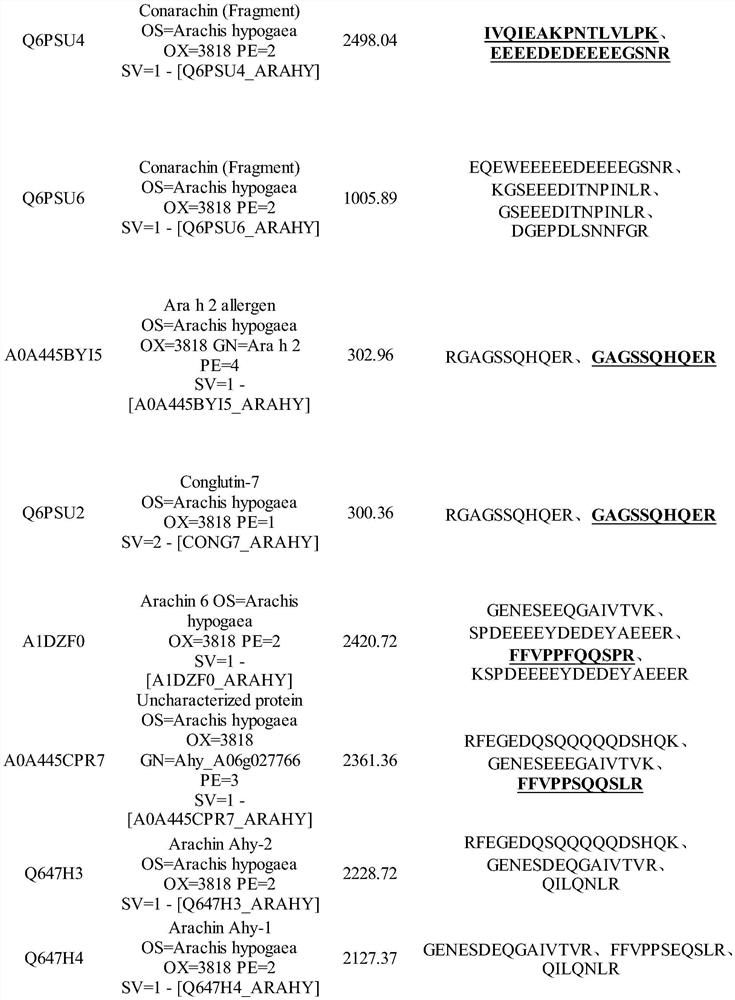
Method for detecting allergen in high oleic acid peanuts by mass spectrometry
PendingCN113514574ASolve the shortcomings of not being able to directly detect allergenic proteinsImprove accuracyComponent separationPeanut food allergyProtein
The invention provides a method for detecting an allergen in high oleic acid peanuts by using mass spectrometry. The method comprises the following steps: performing mass spectrometry on a high oleic acid peanut extract by using a mass spectrometry parallel reaction monitoring method to obtain protein information; comparing and screening the obtained protein information with bioinformatics data in a UniProt database to obtain the allergenic protein related to peanut allergy; and screening out a specific peptide fragment in the sensitization protein through a screening condition. The screening conditions comprise: 1) no modification; 2) the number of spectrums matching the amino acid sequence in the spectrum database is greater than 3; 3) the sequence only exists in one or two proteins; 4) the number of amino acids is 7 to 20; and 5) the peptide fragment does not contain cysteine and methionine. The method can be used for qualitatively and quantitatively detecting the peanut allergenic protein and is relatively high in accuracy; the protein and the peptide fragment can be confirmed, the corresponding peptide fragment can be specifically screened out, and a foundation is laid for subsequently detecting the peanut allergen by using an isotope dilution method.
Owner:CHINA AGRI UNIV
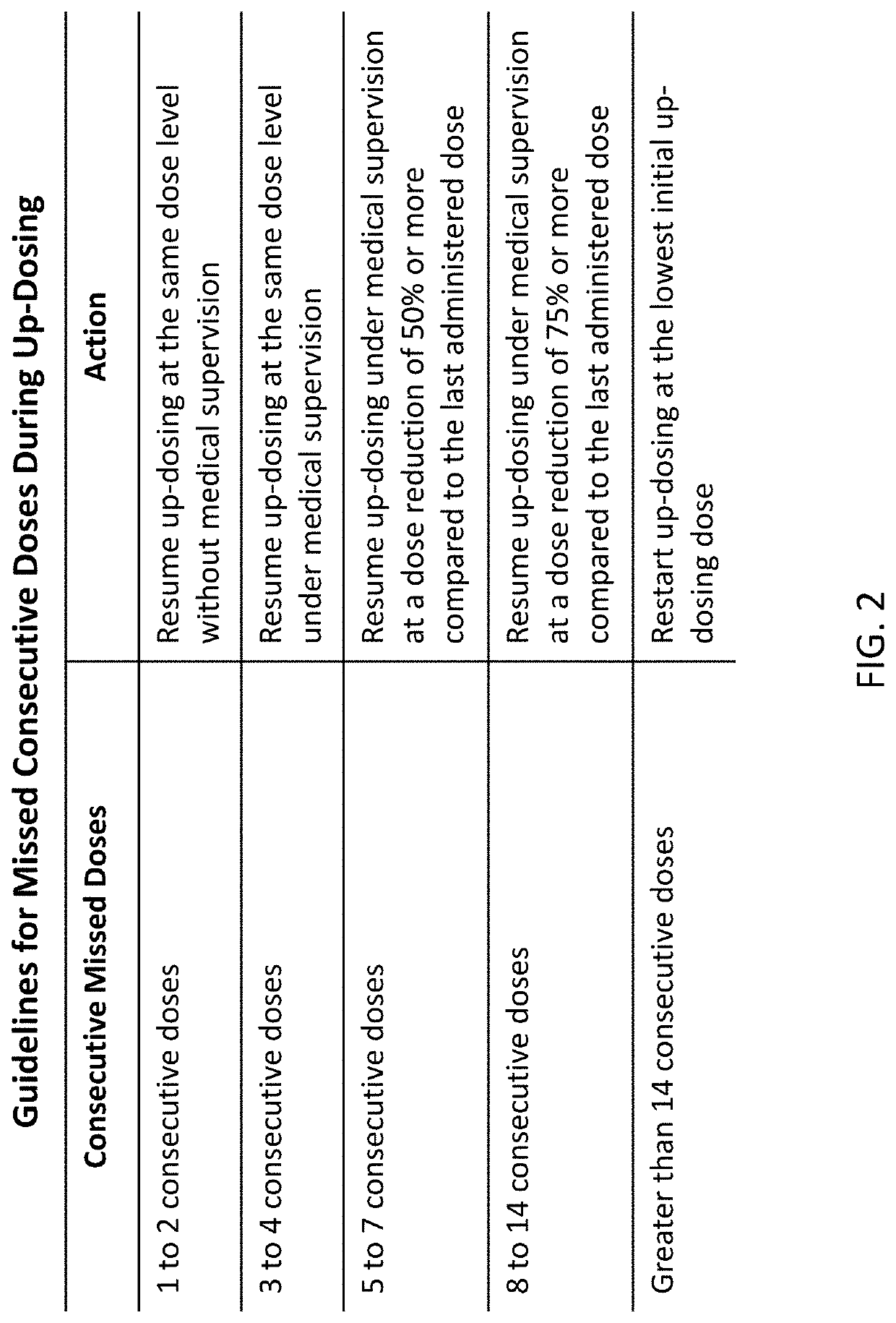
Peanut oral immunotherapy dosing schedule for missed doses
PendingUS20200230206A1Peptide/protein ingredientsPharmaceutical delivery mechanismPeanut food allergyOral medication
The present disclosure relates to improved oral immunotherapy methods for treating peanut allergy. In certain embodiments, the disclosure provides methods for continuing an oral immunotherapy for the treatment of a peanut allergy after missing a scheduled oral administration of one or more consecutive doses of a pharmaceutical composition comprising peanut protein.
Owner:SOC DES PROD NESTLE SA
Therapeutic neutralization antibodies for the treatment of peanut allergy
PendingUS20220348643A1Easy to removeInhibition releaseVaccinesImmunoglobulins against plantsPeanut food allergyAntiendomysial antibodies
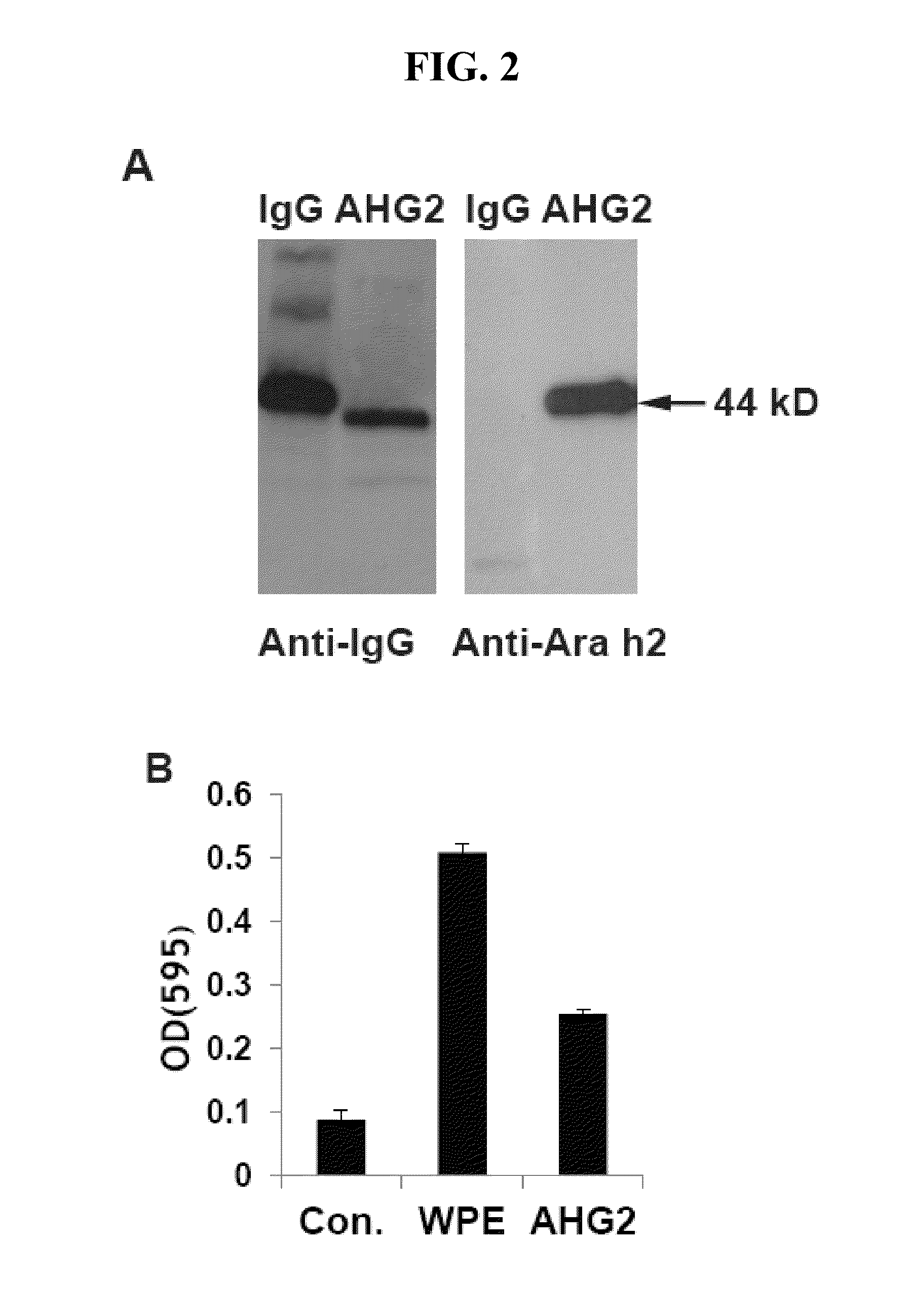
The invention provides anti-Ara h 2 antibodies (e.g., an anti-Ara h 2 neutralizing antibody) and methods of using the same, e.g., for treating and / or preventing peanut allergy or sensitivity. Also provided herein are anti-Ara h 2 antibodies and methods of using the same, e.g., for diagnostics and methods of monitoring peanut oral immunotherapy.
Owner:MASSACHUSETTS INST OF TECH +1
Treatment and/or prevention of peanut induced anaphylaxis
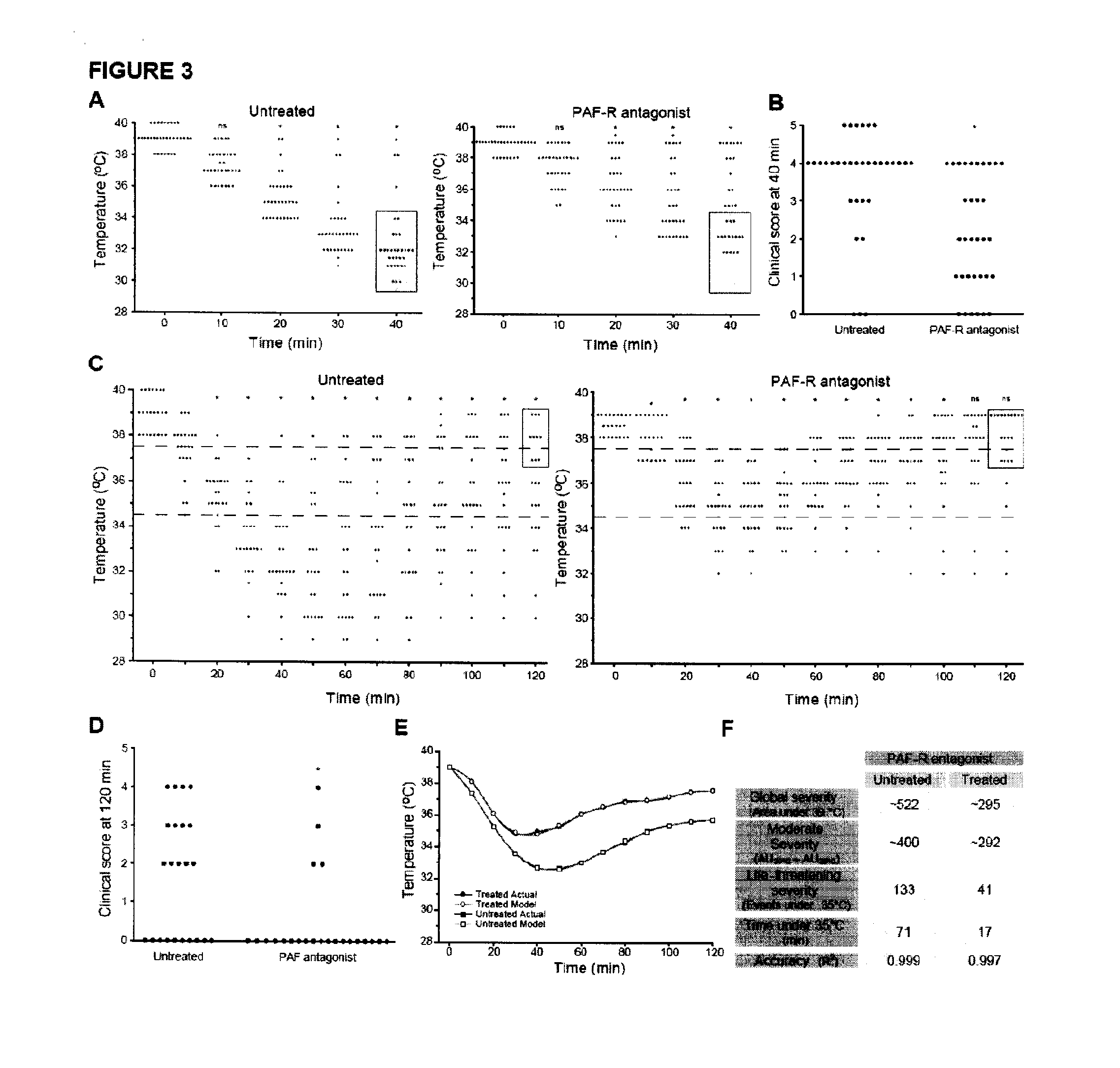
InactiveUS20110217290A1Reduces prolongedReduces life-threatening peanut-induced anaphylactic reactionBiocidePeptide/protein ingredientsPeanut food allergyPlatelet
Compositions and methods for the treatment of food allergies, particularly peanut allergy are provided. The compositions and methods are based on the observation that agents which interfere with the association of platelet activating fact (PAF) with its receptor (PAF-R) significantly reduce the severity and duration of anaphylaxis. Combination therapy with a PAF inhibitor and a histamine inhibitor provided an increased beneficial effect compared to treatment alone.
Owner:JORDANA MANEL +2
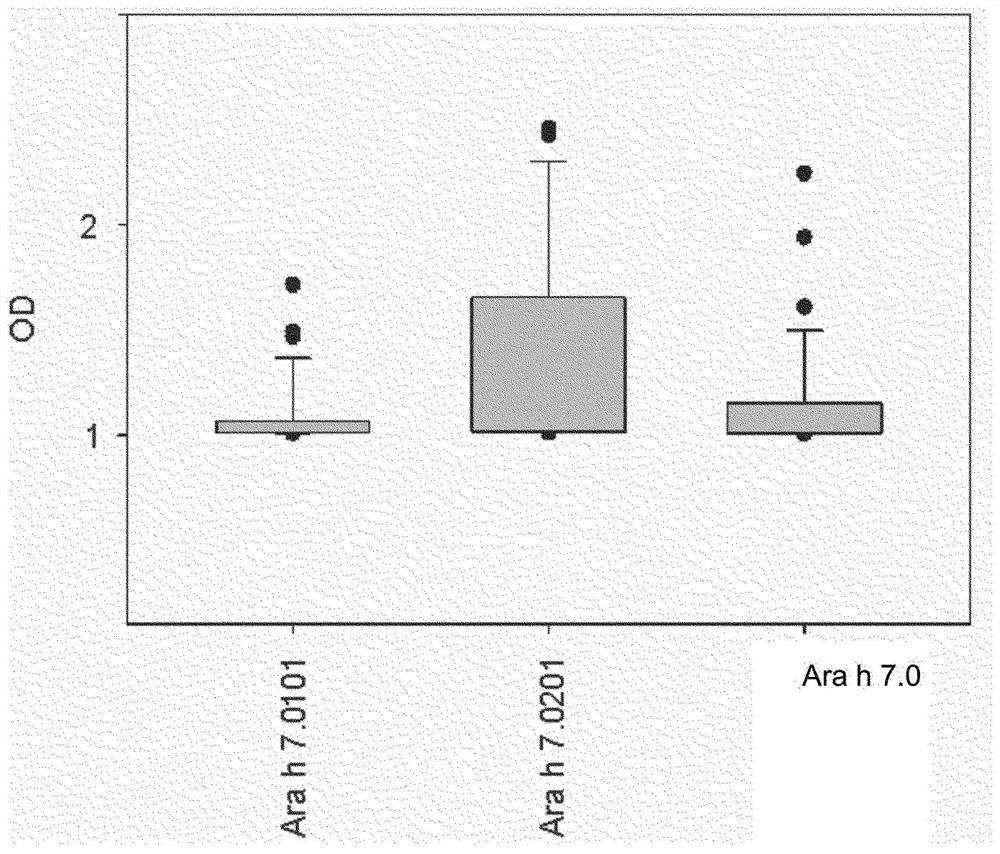
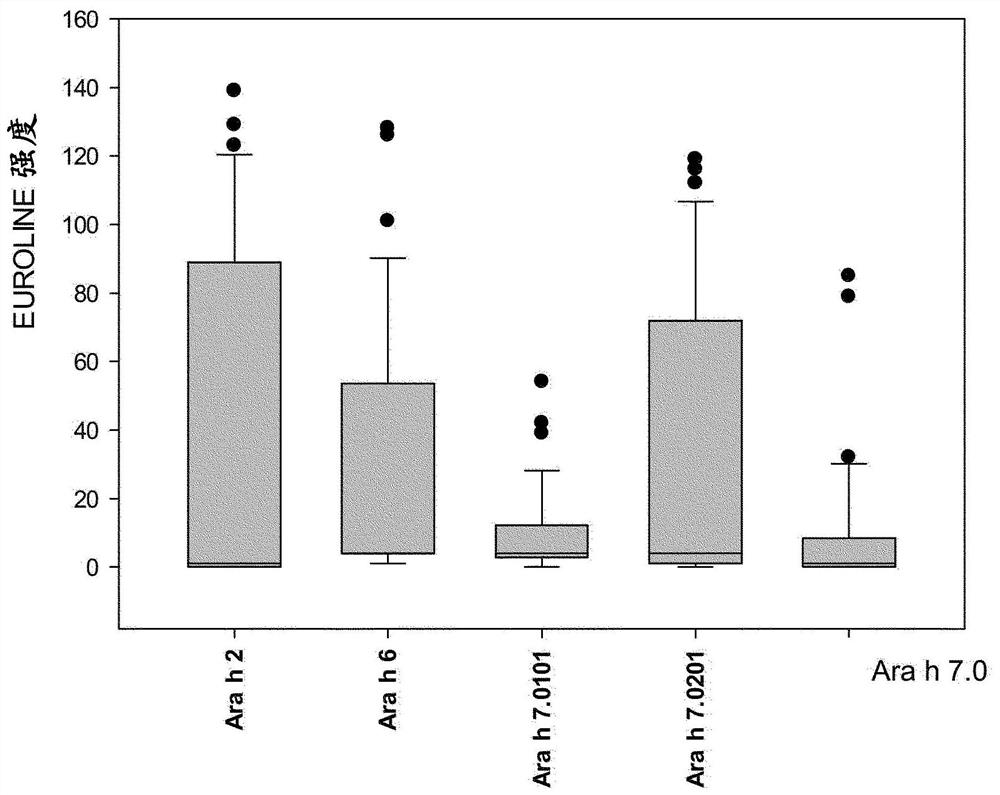
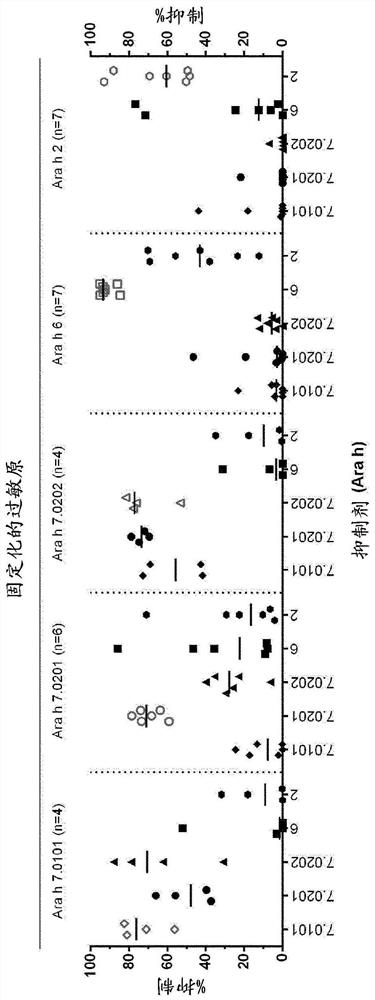
An improved assay for the diagnosis of peanut allergy
The invention relates to an improved assay for the diagnosis of peanut allergy. The present invention relates to a diagnostically useful carrier comprising a means for specifically capturing an antibody to Ara h 7 isotype 7.0201 in a sample from a subject; a method comprising the step detecting in a sample from a subject the presence or absence of an antibody to Ara h 7 isotype 7.0201 and a pharmaceutical composition comprising Ara h 7 isotype 7.0201 or a variant thereof.
Owner:EUROIMMUN MEDIZINISCHE LABORDIAGNOSTIKA
Treatment of peanut allergy
ActiveUS10898569B2Prevention and treatment of peanut allergyEnhance immune responseSsRNA viruses positive-senseAllergen ingredientsPeanut food allergyPharmaceutical drug
Owner:ALLERGY THERAPEUTICS UK
Gastrointestinal release capsule for use in a method for desensitising and/or inducing tolerance in a patient with a peanut allergy
ActiveUS11185567B2Risk minimizationAvoid contactOrganic active ingredientsInorganic non-active ingredientsBiotechnologyPeanut food allergy
Gastro-intestinal release capsule intended for oral use in a method for desensitizing and / or inducing tolerance in a peanut-allergic subject or, alternatively, for diagnosing a peanut allergy in a subject. The capsule has a shell and a core, the core has a composition of peanut including peanut, at least one oil, at least one first pulverulent excipient and, optionally, at least one second prebiotic excipient. The capsule is provided and ingested in an unopened form.
Owner:CENT HOSPITALER & UNIV DE CLERMONT FERRAND +1
Treatment of peanut allergy
ActiveUS20190134189A1Prevention and treatment of peanut allergyIncrease in generated immune responseSsRNA viruses positive-senseAllergen ingredientsPeanut food allergyImmunogenicity
Owner:ALLERGY THERAPEUTICS UK
Gastrointestinal release capsule for use in a method for desensitising and/or inducing tolerance in a patient with a peanut allergy
ActiveUS20200038466A1Promising immune-modulator propertyDesensitization of subjectOrganic active ingredientsInorganic non-active ingredientsBiotechnologyPeanut food allergy
Gastro-intestinal release capsule intended for oral use in a method for desensitizing and / or inducing tolerance in a peanut-allergic subject or, alternatively, for diagnosing a peanut allergy in a subject. The capsule has a shell and a core, the core has a composition of peanut including peanut, at least one oil, at least one first pulverulent excipient and, optionally, at least one second prebiotic excipient. The capsule is provided and ingested in an unopened form.
Owner:CENT HOSPITALER & UNIV DE CLERMONT FERRAND +1
Methods for improving the quality of life of a patient with a peanut allergy
ActiveUS20200368304A1Improve the quality of lifePharmaceutical delivery mechanismImmunological disordersPeanut food allergyOral immunotherapy
The present disclosure relates to methods of improving the quality of life of a subject having a peanut allergy. In certain embodiments, the disclosure provides methods for improving the quality of life of a subject having a peanut allergy by administering a peanut composition according to an oral immunotherapy schedule. In certain embodiments, the disclosure provides methods for improving the quality of life of a subject having a peanut allergy by informing the subject they are to be, or are being, administered a peanut composition according to an oral immunotherapy schedule.
Owner:SOC DES PROD NESTLE SA
Immunotherapeutic molecules and uses thereof
The present invention relates generally to molecules such as peptides, polypeptides and proteins which interact immunologically with T lymphocytes in subjects having peanut allergy, or allergy to other tree nuts, and genetic sequences encoding same. These molecules are preferably immunointeractive with T cells in subjects having an allergy to the Ara h 1 allergen. The molecules of the present invention are useful in the development of diagnostic, therapeutic and prophylactic agents for conditions characterised by an aberrant, inappropriate or otherwise unwanted immune response to Ara h 1 or derivative or homologue thereof.
Owner:ARAVAX
Methods of oral immunotherapy
ActiveUS11369676B2Reduce incidenceReduce riskAllergen ingredientsPharmaceutical delivery mechanismPeanut food allergyOral immunotherapy
The present disclosure relates to improved oral immunotherapy methods for treating food allergies, and particularly peanut allergy. Biomarkers, such as a level of peanut-specific IgE and / or peanut-specific IgG4s are used by the methods described herein. Such methods include a method of treating a subject for a peanut allergy, methods of assessing the suitability of a treatment for a peanut allergy, a method of evaluating a symptom in a subject during the course of treatment of a peanut allergy, a method of monitoring treatment for a peanut allergy in a subject, a method of reducing the risk or incidence of an adverse event in a subject receiving treatment for a peanut allergy, a method of adjusting a dose of an allergenic peanut composition, and a method of assessing a likelihood of an allergic reaction that requires administration of epinephrine to a subject receiving treatment for a peanut allergy.
Owner:SOC DES PROD NESTLE SA
Systemic allergic response risk assessment in peanut oral immunotherapy
PendingUS20220221468A1Pharmaceutical delivery mechanismAllergen ingredientsPeanut food allergyOral immunotherapy
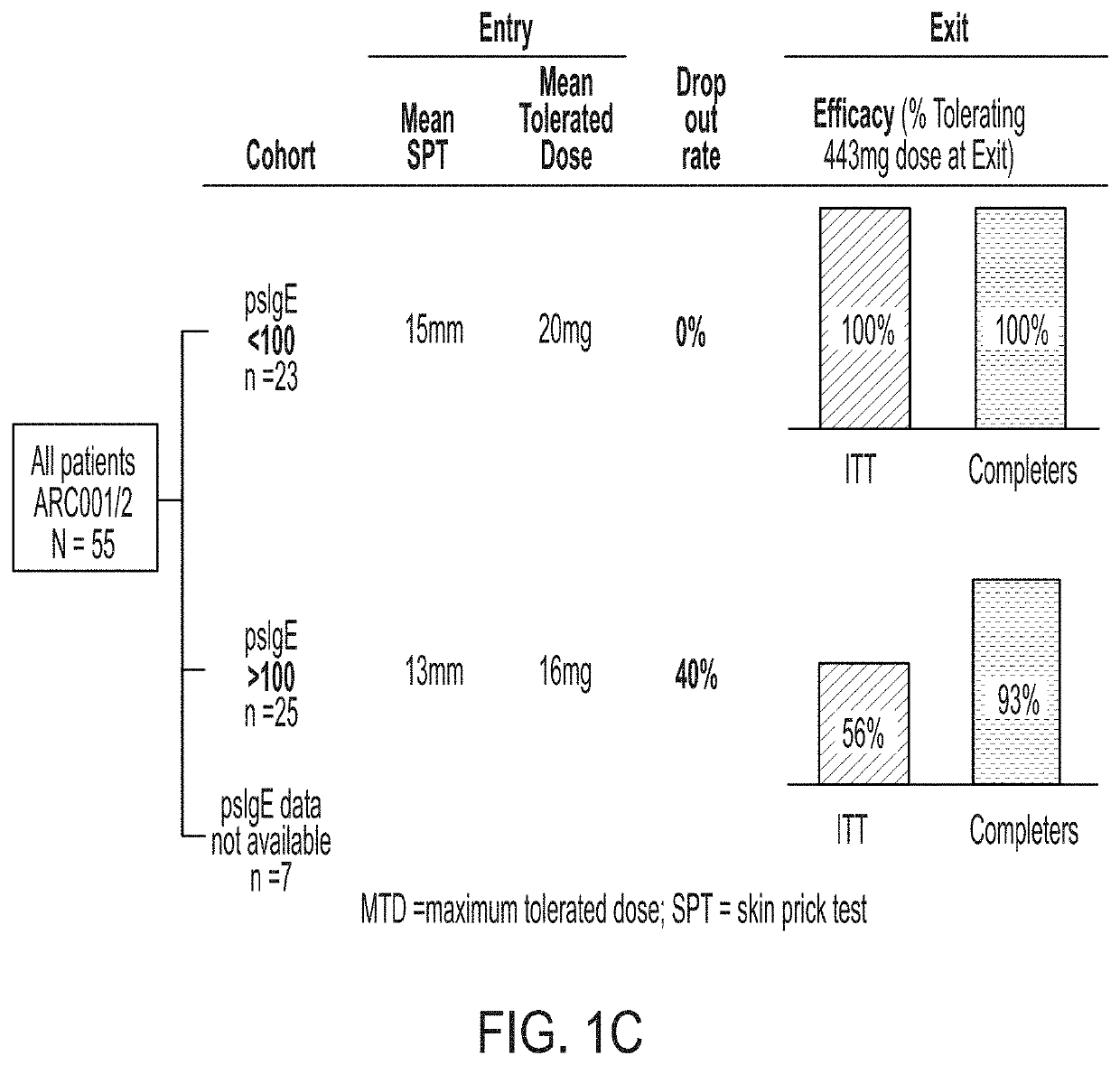
Described herein are methods of assessing risk of a systemic allergic response in a subject being treated for a peanut allergy by an oral immunotherapy. The oral immunotherapy includes administering to the subject a composition comprising peanut protein according to an oral immunotherapy schedule comprising an up-dosing phase and a maintenance phase. The methods can include obtaining a peanut-specific IgE level when the subject tolerates the dose of 1000 mg or more peanut protein; and assessing the risk of a systemic allergenic response in the subject based on the obtained peanut-specific IgE level.
Owner:AIMMUNE THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com