Treatment for peanut allergy
A technology for peanut allergy and peanut powder, applied in allergic diseases, antibody medical ingredients, medical preparations containing active ingredients, etc., can solve problems such as increased risk of unintentional ingestion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0104] experiment
[0105] method 1
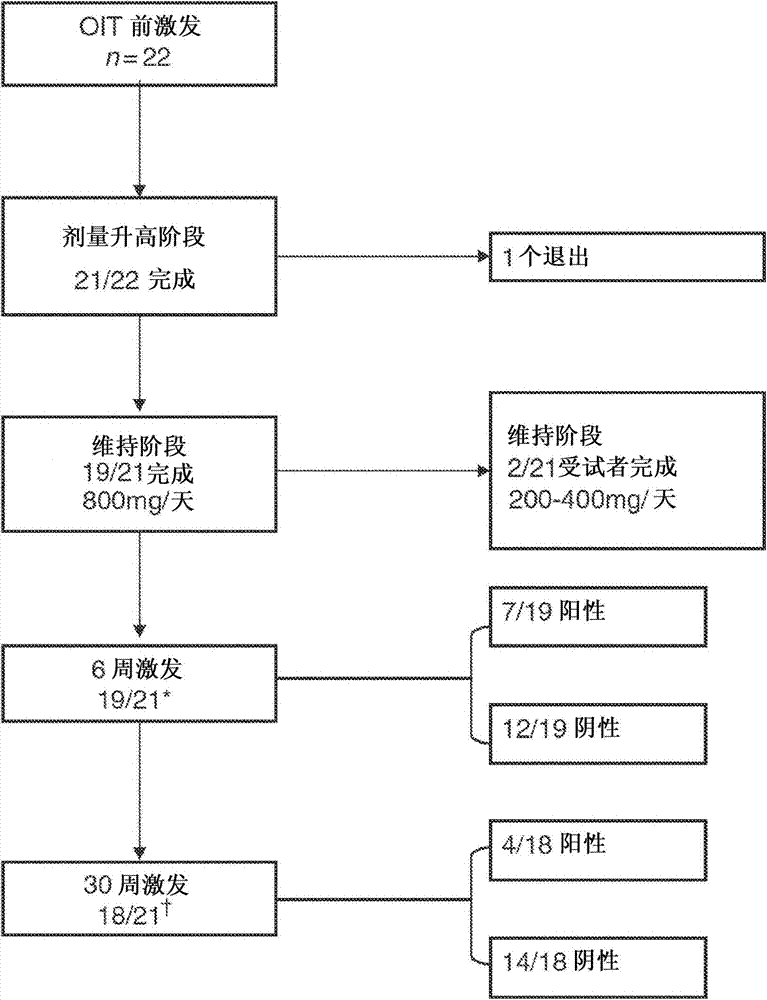
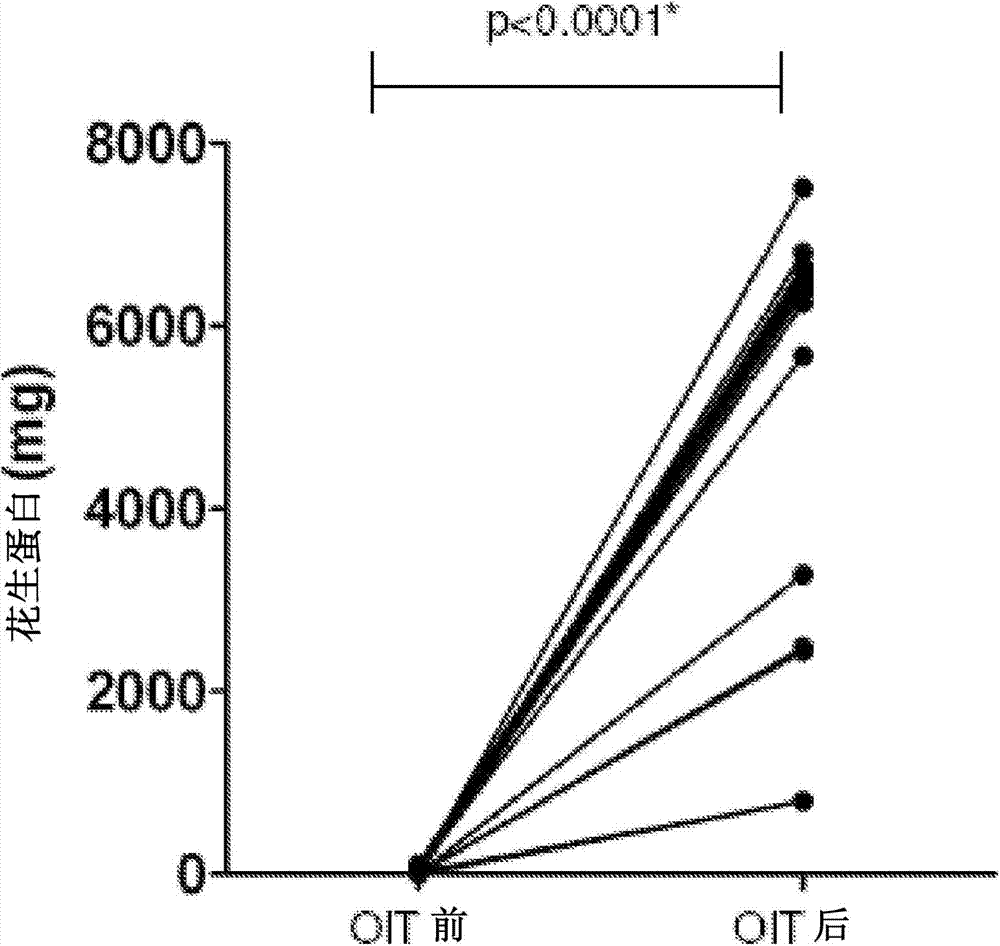
[0106] The study was approved by the Local Ethics and Research and Development Committees and written informed consent was given to each family. Inclusion criteria were positive peanut oral (immunization) challenge and presence of peanut-specific IgE in children 4-18 years of age. Severe immunodeficiency and inability to comply with the study protocol were exclusion criteria. 22 children were selected. Children with a history of anaphylaxis following peanut ingestion were included.
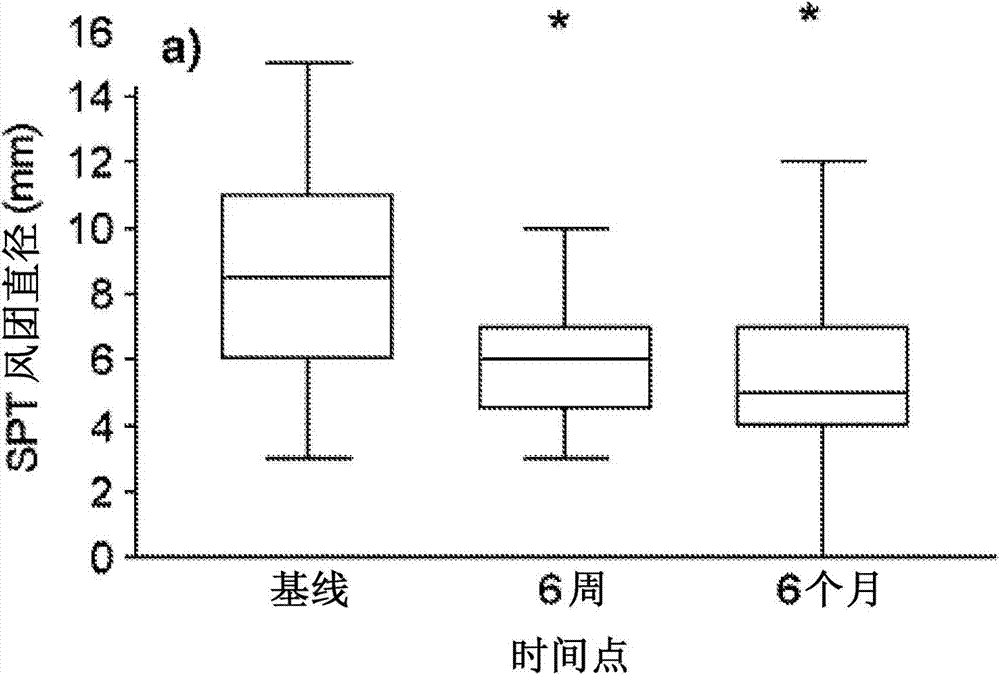
[0107] Skin prick test (SPT) (peanut extract, saline negative control and histamine 10mg / mL positive control; single point lancet; ALK-Abello, Denmark ), peanut SPT was interpreted as positive when the wheal diameter was at least 3 mm larger than the negative control. Serum analysis was performed for whole peanut and Ara h2-specific IgE (CAP-Systems FEIA; Phadia, Uppsala, Sweden).
[0108] Double-blind placebo-controlled food challenge (DBPCFC)
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com