Nucleic acids for treatment of peanut allergies
A peanut allergen, nucleic acid molecule technology, applied in the fields of molecular biology and medicine, can solve the problem of varying degrees
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1: General Materials and Methods
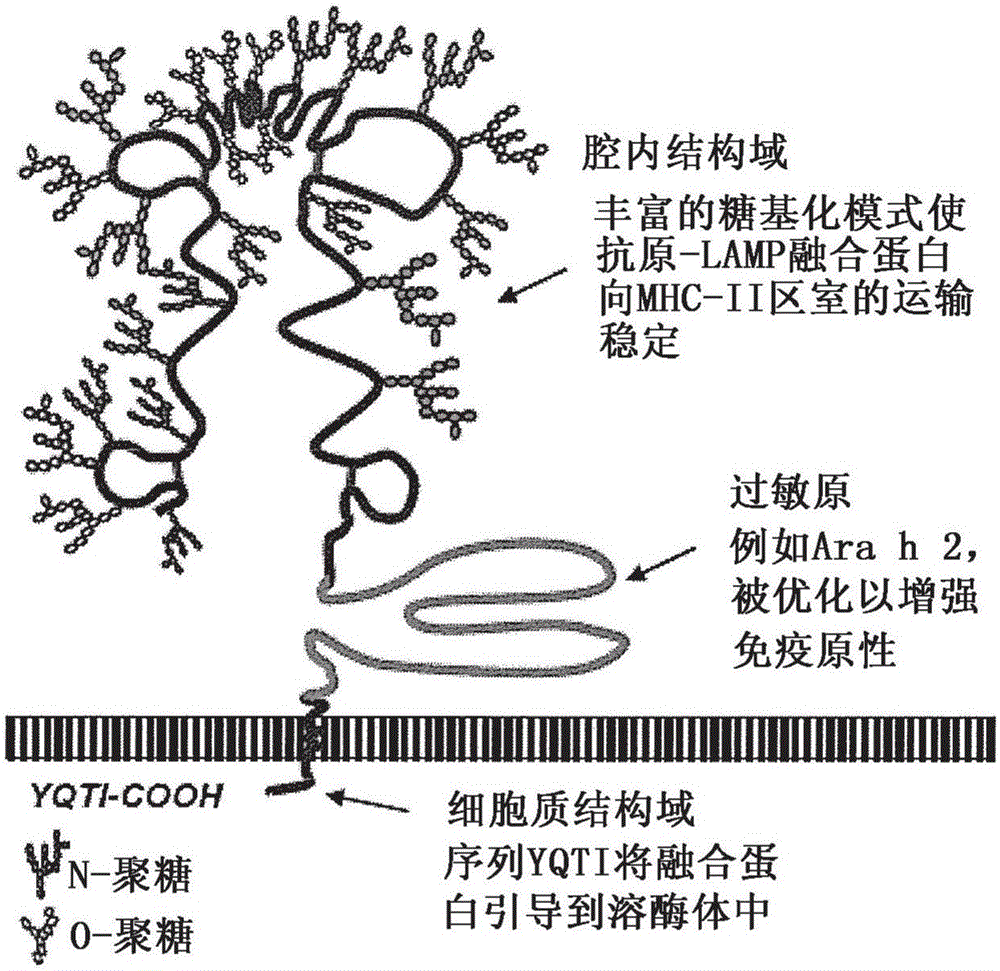
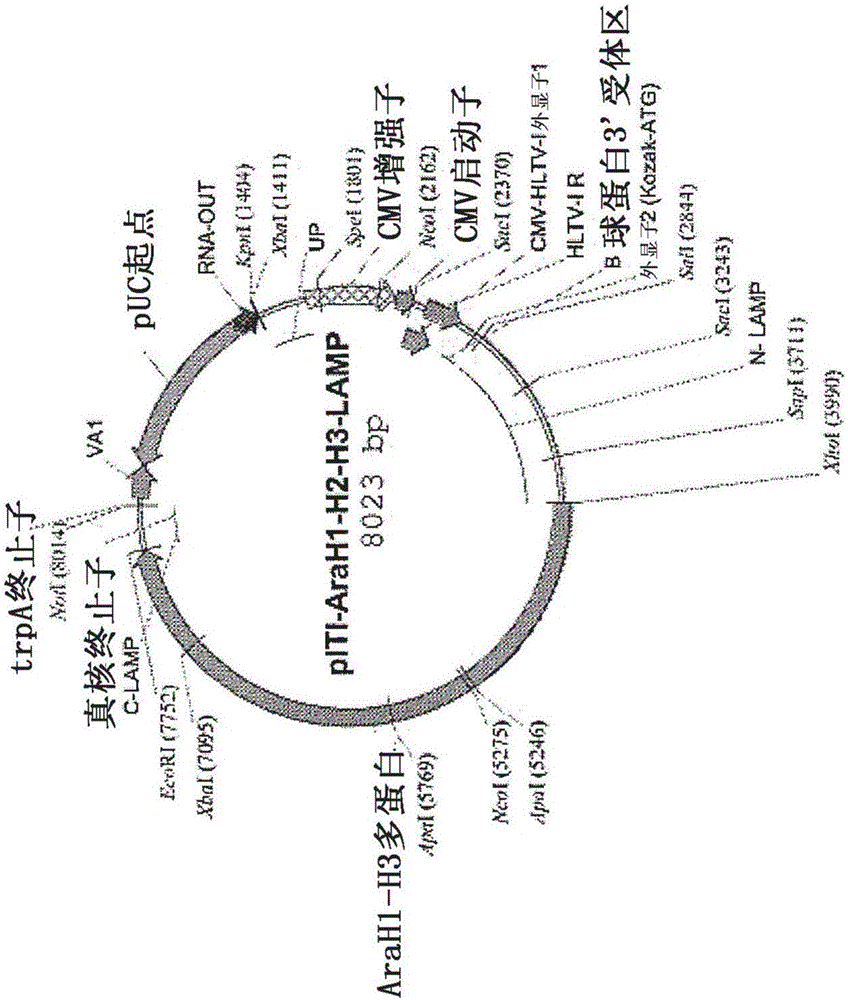
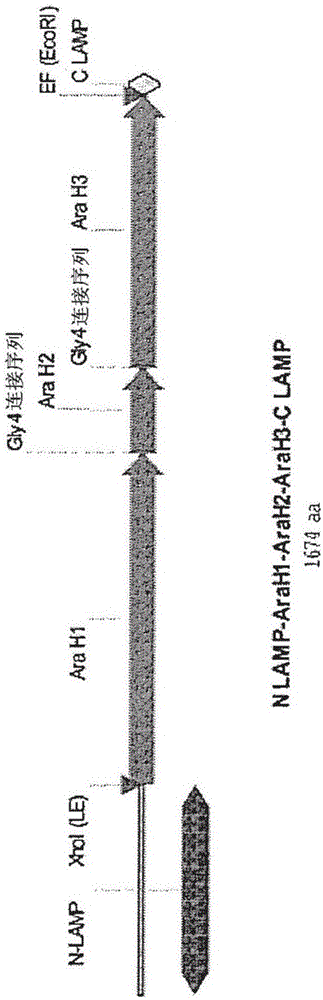
[0126] Genetic sequences encoding the peanut allergens Ara H1, Ara H2 and Ara H3 were prepared as native sequences (control plasmid) and as chimeras with human LAMP-1 (experimental plasmid), wherein each sequence was inserted in the LAMP Between the luminal domain and the transmembrane domain. Previous studies have shown that antigenic sequences must be optimized for human use, thus removing all non-essential or deleterious elements (cryptic splice sites, secondary RNA / DNA structures, secondary ORFs) in order to maximize RNA stability sex and protein expression. AraH1-LAMP comprises SEQ ID NO:15. AraH2-LAMP comprises SEQ ID NO:12. The final optimized sequence was chemically synthesized and inserted into the LAMP open reading frame (ORF) of the antibiotic-free pDNA-VACC-ultra vector (Nature Pharmaceuticals, Lincoln, NE). Chimeric protein expression for each plasmid was determined by transfection of NIH3T3 cells followed by...
Embodiment 2
[0135] Example 2: ARA-LAMP Prevention Study
[0136] The prophylactic effectiveness of DNA constructs comprising the peanut allergens Ara H1, Ara H2 and / or Ara H3 was tested in a mouse model. The polyvalent LAMP plasmids encoding the peanut allergens Ara H1, Ara H2 and Ara H3 (AraH1 / H2 / H3-LAMP generated according to Example 1) were mixed with a three-plasmid mixture each encoding a single peanut allergen (according to Example 1). 1 prepared AraH1-LAMP, AraH2-LAMP and ARAH3del-LAMP) for comparison. BALB / c mice were immunized once a week for three weeks with 50 μg of a single multivalent peanut plasmid or 50 μg of each individual plasmid by intradermal (ID) or intramuscular (IM) injection. IgG1 and IgG2a antibody titers were determined by ELISA 5 weeks after the last immunization. Multivalent plasmids were found to be allergenic, but the magnitude of the antibody response was lower than a single allergen delivery in multiple plasmids ( Figure 5 and 6 ). Without wishing t...
Embodiment 3
[0141] Example 3: ARA-LAMP therapeutic study
[0142] Experiments were also performed to determine the ability of the DNA vaccines of the present disclosure to provide therapeutic treatment. A representative scheme is shown in the Figure 17 wherein the mice were first sensitized with peanut butter and cholera toxin, and then the ARA-LAMP-vax triple plasmid (AraH1-LAMP, AraH2-LAMP and Ara-H3del-LAMP prepared according to Example 1) composition treat. Figure 18 Shown are IgE antibody levels in the weeks preceding vaccine treatment with the three-plasmid mixture ARA-LAMP-vax, each encoding a single peanut allergen.
[0143] When multivalent DNA vaccines were used, IgE antibody levels decreased at week 15 after vaccine treatment ( Figure 19 ). Anaphylaxis challenge results (symptom score, Figure 20 , Figure A; body temperature, Figure 20 , Figure B; Figure 21 ) showed that administration of the ARA-LAMP-vax three-plasmid composition resulted in less severe symptoms ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com