Antibody prodrug and preparation method and application thereof
A technology of prodrugs and antibodies, applied in the direction of antibodies, anti-tumor drugs, drug combinations, etc., can solve the problems of lack of targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Construction of targeted antibody prodrugs
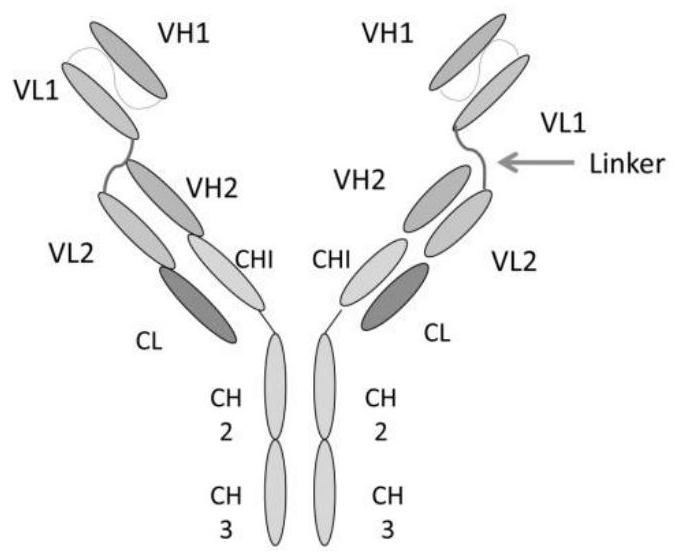
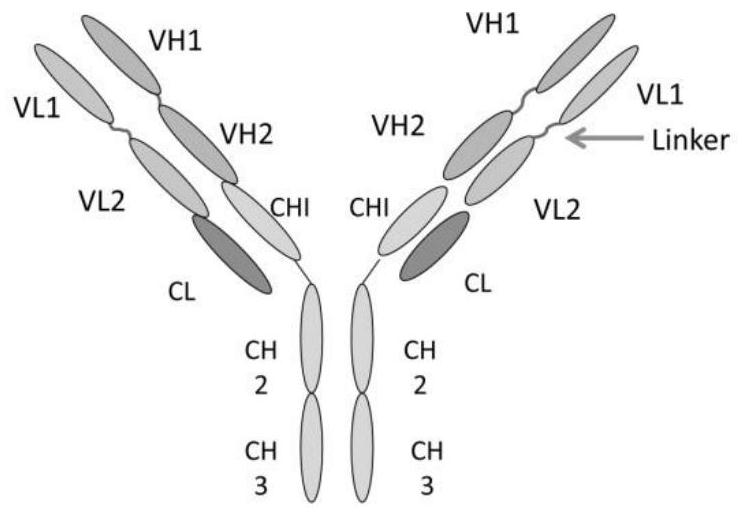
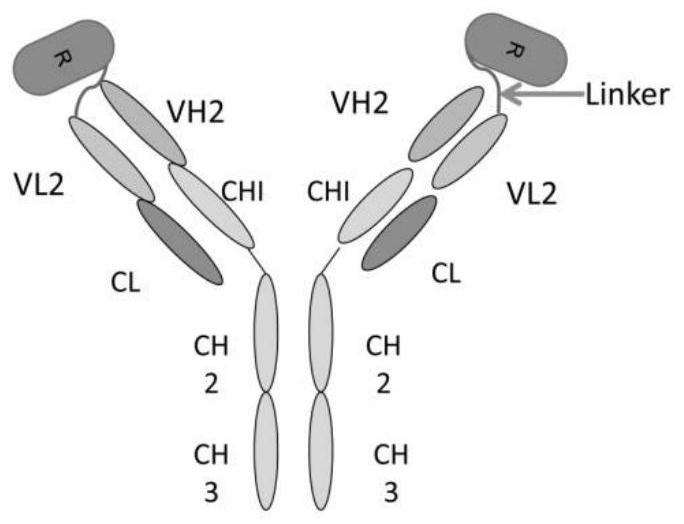
[0054] Use genetic engineering technology to synthesize part A with targeted protein gene sequence, such as VEGF single-chain antibody gene sequence, the light chain variable region has the nucleotide sequence shown in SEQ ID NO:1 and the amino acid shown in SEQ ID NO:2 Sequence, the heavy chain variable region has the nucleotide sequence shown in SEQ ID NO:3 and the amino acid sequence shown in SEQ ID NO:4.
[0055] Using genetic engineering technology to synthesize part C of the antibody gene sequence with therapeutic effect, such as the complete antibody gene sequence of EGFR, its light chain has the nucleotide sequence shown in SEQ ID NO:5 and the amino acid sequence shown in SEQ ID NO:6, Its heavy chain has the nucleotide sequence shown in SEQ ID NO:7 and the amino acid sequence shown in SEQ ID NO:8.
[0056] The connecting peptide part B connecting part A and part C has the amino acid sequence shown in ASLSG...
Embodiment 2
[0059] Example 2: Cell culture, expression and purification of targeted antibody prodrugs
[0060] The CHO host cells transfected with the targeting antibody prodrug were cultured, expressed and purified without serum to obtain the targeting antibody prodrug CMAB0301.
[0061] Part A of the targeted antibody prodrug CMAB0301 is a VEGF single-chain antibody whose light chain variable region has the amino acid sequence shown in SEQ ID NO: 2, and the heavy chain variable region has the amino acid sequence shown in SEQ ID NO: 4; part B is a connecting peptide containing a urokinase-type plasminogen activation substrate, which has the amino acid sequence shown in ASLSGRSDNHGSAS; part C has the amino acid sequence shown in SEQ ID NO: 6 for the light chain, and the amino acid sequence shown in SEQ ID NO: 8 for the heavy chain. Whole antibody to EGFR with amino acid sequence shown.
[0062] The targeted antibody prodrug CMAB0301 adopts basic culture and supplementary culture methods ...
Embodiment 3
[0070] Example 3: In vitro activity detection of targeted antibody prodrugs
[0071] The targeting antibody prodrug CMAB0301 was obtained by the above method, and the binding and affinity detection of VEGF and EGFR were performed.
[0072] (1) VEGF binding assay (ELISA):
[0073] Coat the recombinant VEGF165 antigen (1 μg / ml) on a 96-well ELISA plate, add 100 μl to each well overnight at 4°C; block the ELISA plate with PBS containing 10% BSA at 37°C for 1 h; Add CMAB0301 serially diluted from high concentration, incubate at 37°C for 1 h, wash with PBS containing 0.1% Tween-20 three times; add horseradish peroxidase-coupled goat anti-human F(ab')2 The secondary antibody (1:1,000) was incubated for 30 min; TMB chromogenic solution was added, the color was developed for 10 min, the reaction was terminated with 1M sulfuric acid, and the absorbance value (OD value) was measured at 450 nm with a microplate reader.
[0074] The binding ability of targeting antibody prodrug CMAB0301...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap