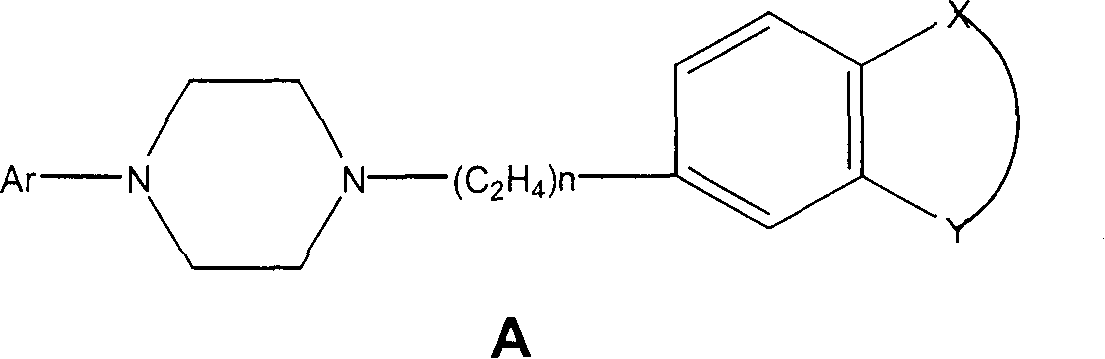
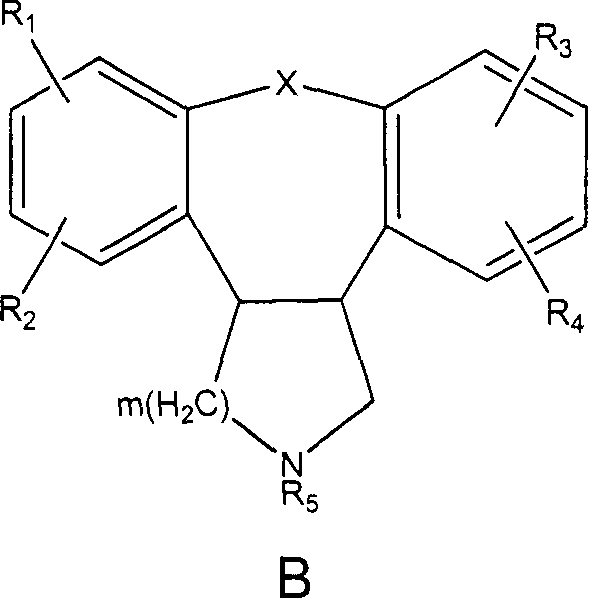
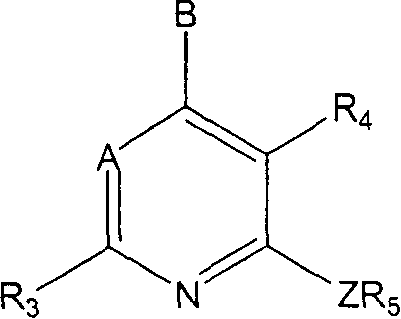
Therapeutic combinations of atypical antipsychotics with corticotropin releasing factor antagonists
A technology of antipsychotic drugs and adrenal cortex, which can be used in drug combinations, nervous system diseases, and pharmaceutical formulations, and can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0577] A pharmaceutical composition is prepared by combining ziprasidone with a CRF antagonist, either (a) 4-(1-ethyl-propoxy)-3,6 in a pharmaceutically acceptable carrier -Dimethyl-2-(2,4,6-trimethylphenoxy)-pyridine, (b) (3,6-dimethyl-2-(2,4,6-trimethyl-benzene Oxy)-pyridin-4-yl)-(1-ethyl-propyl)-amine, (c)(3,6-dimethyl-2-(4-chloro-2,6-dimethyl- Phenoxy)-pyridin-4-yl)-(1-ethyl-propyl)-amine, or (d) 5-(1-ethyl-propoxy)-7-methyl-1-( 2,6-Dimethyl-4-chlorophenyl)-1-4-dihydro-2H-3-oxa-1,8-naphthyridine. The composition comprises corresponding amounts of ziprasidone and CRF antagonist to provide about 20 mg to about 160 mg of ziprasidone and about 0.1 to 100 mg of CRF antagonist on a daily basis. The composition is administered to patients once a day, twice a day, three times a day or four times a day for treating schizophrenia.
Embodiment 2
[0579] Combination administration of ziprasidone and CRF antagonists
[0580] A prospective, open-label, randomized, scaled-dose multicentre study was performed comparing IM (intramuscular injection) with and without a CRF antagonist at the dose of CRF antagonist described in Example 1. ) Effectiveness of ziprasidone in improving anxiety and psychosis in patients with psychotic disorders. Ziprasidone is administered by intramuscular injection in doses of 10 or 20 mg, with additional daily doses up to a maximum of 40 mg if necessary.
[0581] About half of the ziprasidone-treated patients received at least one dose of the CRF antagonist of Example 1 during IM therapy. The primary efficacy outcome was the mean change from baseline in scores on the Brief Psychiatric Rating Scale (BPRS), CGI-S, and CGI-Improvement (CGI-I) scores. BPRS, CGI-S, and CGI-I were assessed every 24 hours during IM treatment and at baseline at the end of day 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com