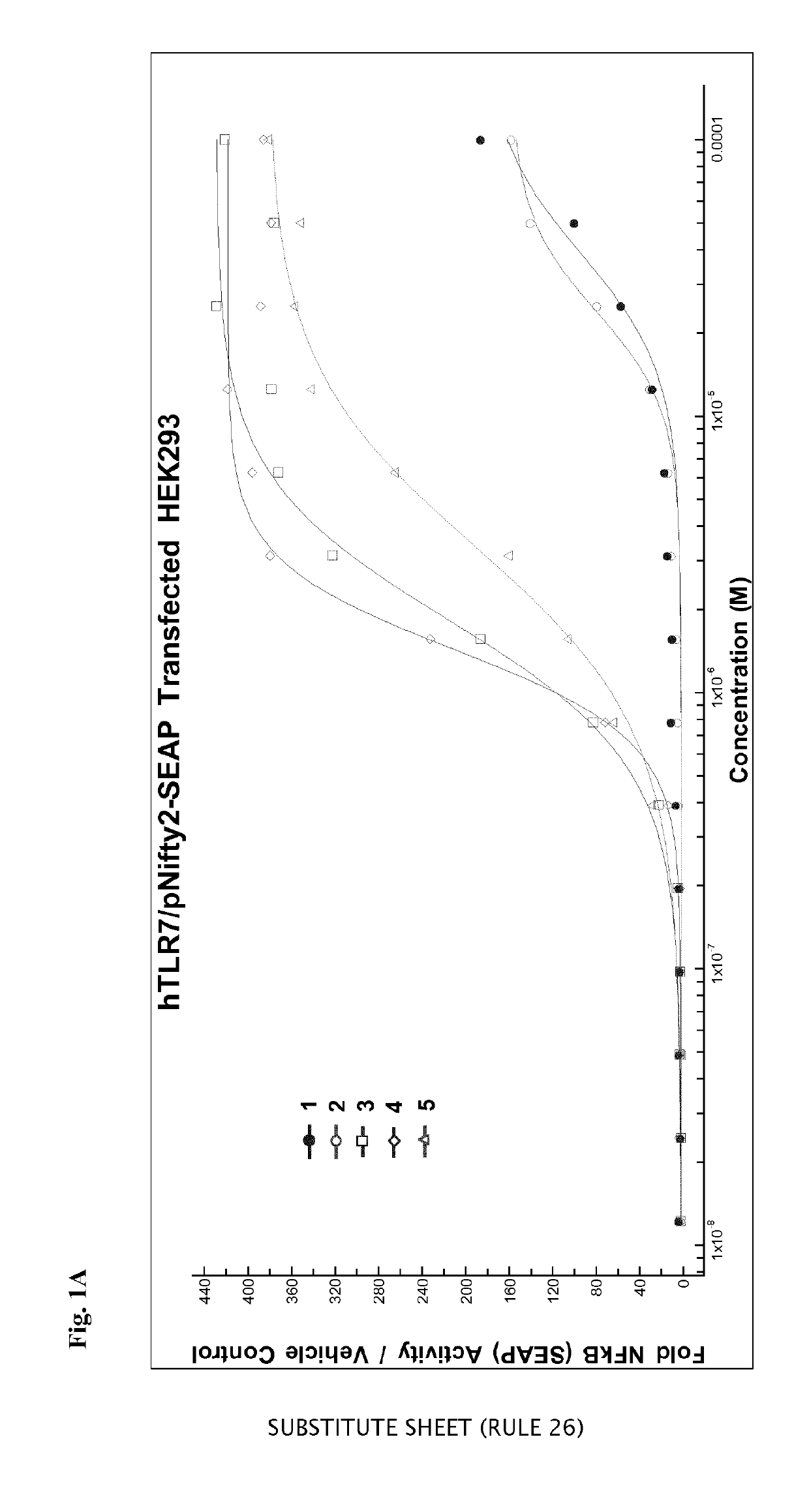
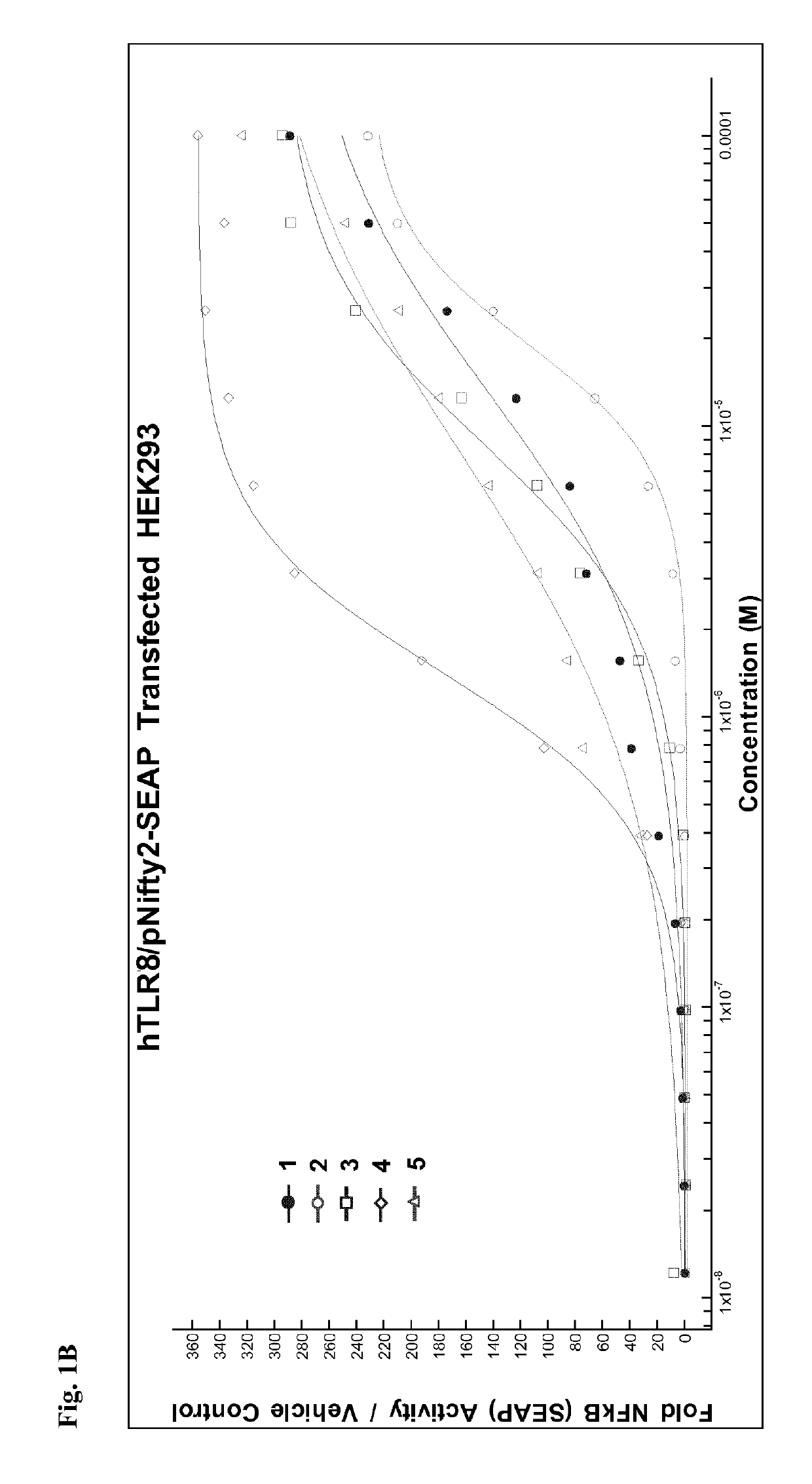
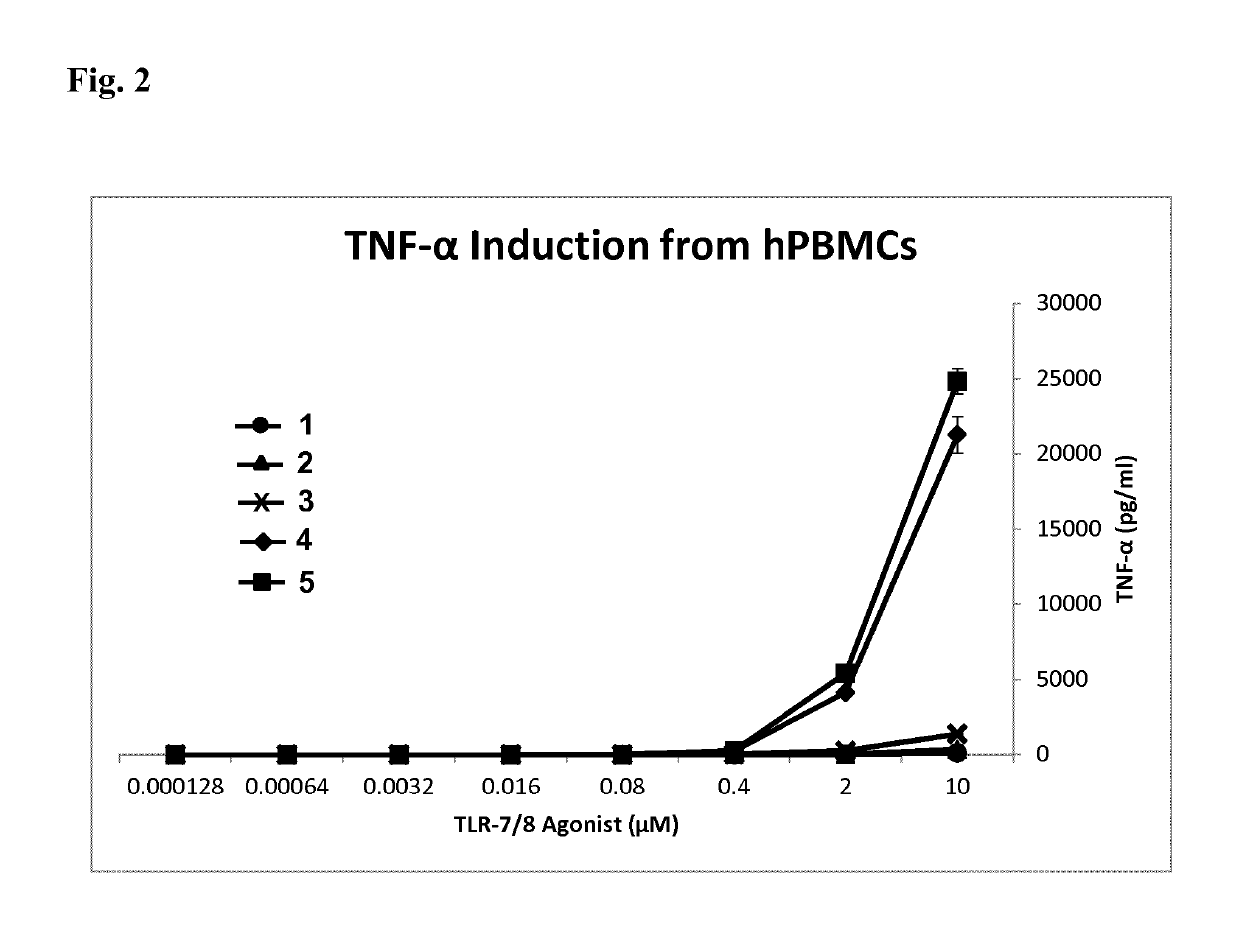
Pegylated imidazoquinolines
a technology of imidazoquinoline and pegylated imidazoline, which is applied in the field of tolllike receptor 7 (tlr7) and tolllike receptor 8 (tlr8) agonists, can solve the problems of imiquimod's relatively low interferon-inducing activity, the decline of vaccine potency, and the difficulty in the design of vaccine adjuvants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
rocedure for the Synthesis of Compound of Formula (I)
[0214]
[0215]Imidazoquinoline monophosphate glycerides (I) were prepared by[0216]a) reacting a compound of formula (III) (prepared according to methods known in the art, Bioorg Med Chem, 2013, 21, 3066; Chem Bio Chem, 2012, 13, 2331; Chem Eur J, 2006, 6, 111), with a phosphordiamidite reagent of formula (II) (commercially available) according to methods known in the art;[0217]b) reacting a compound of formula (IV) (not isolated) in-situ with an imidazoquinoline of formula (VII) (Gerster et al., J. Med. Chem., 2005, 48, 3481; Izumi et al., Bioorg Med Chem, 2003, 11, 2541) according to methods known in the art;[0218]c) oxidizing a compound of formula (VIII) and removing the protecting group according to methods known in the art to produce a compound of formula (I).
[0219]A compound of formula (III) (2.0 eq) and 2-cyanoethyl N,N,N′,N′-tetraisopropylphosphordiamidite of formula (II) (2.1 eq) were dissolved in anhydrous methylene chlorid...
example 2
of 4-amino-1-[2-(1,2-dipalmitoyl-sn-glycero-3-ethyleneglycol-phospho)ethyl]-2-n-butyl-1H-imidazo[4,5-c]quinoline. Designated Compound 2 in HEK293 Results
[0220]
[0221]4-amino-1-[2-(1,2-dipalmitoyl-sn-glycero-3-ethyleneglycol-phospho)ethyl]-2-n-butyl-1H-imidazo[4,5-c]quinoline was prepared in 66% yield following the general procedure described in Example 1. 1H NMR (400 MHz, CDCl3 / CD3OD) δ 8.19 (bs, 1H), 7.38 (s, 1H), 7.13 (bs, 1H), 6.93 (bs, 1H), 5.24 (s, 1H), 4.83 (bs, 2H), 4.58 (bs, 2H), 4.39 (dd, 1H), 4.17 (m, 1H), 4.02 (dd, 2H), 3.68 (m, 4H), 2.98 (bs, 2H), 2.31 (dd, 4H), 1.93 (bs, 2H), 1.58 (m, 2H), 1.25 (m, 48H), 1.03 (t, 3H), 0.86 (t, 6H); negative ES TOF-MS calc for [M-H]− 957.6445, found 957.6414.
example 3
of 4-amino-1-[2-(1,2-dipalmitoyl-sn-glycero-3 triethyleneglycol-phospho)ethyl]-2-n-butyl-1H-imidazo[4,5-c]quinoline Designated Compound 3 in HEK293 Results
[0222]
[0223]4-amino-1-[2-(1,2-dipalmitoyl-sn-glycero-3 triethyleneglycol-phospho)ethyl]-2-n-butyl-1H-imidazo[4,5-c]quinoline was prepared in 62% yield following the general procedure described in example 1. 1H NMR (400 MHz, CDCl3 / CD3OD) δ 8.23 (d, 1H), 7.42 (t, 1H), 7.20 (t, 1H), 6.97 (t, 1H), 5.23 (m, 1H), 4.74 (bd, 2H), 4.63 (bd, 2H), 4.34 (dd, 1H), 4.15 (dd, 1H), 4.05 (m, 2H), 3.58-3.71 (m, 12H), 3.04 (bt, 2H), 2.31 (dd, 4H), 1.92 (m, 2H), 1.52-1.59 (m, 6H), 1.26 (m, 48H), 1.04 (t, 3H), 0.88 (t, 6H); negative ES TOF-MS calc for [M-H]− 1045.6970, found 1045.6855.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| TNF-α | aaaaa | aaaaa |
| IFN-α | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap