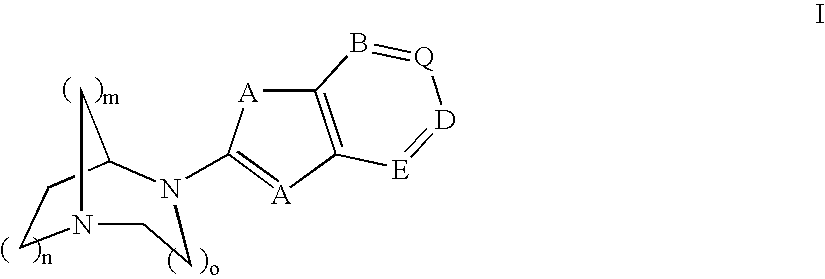
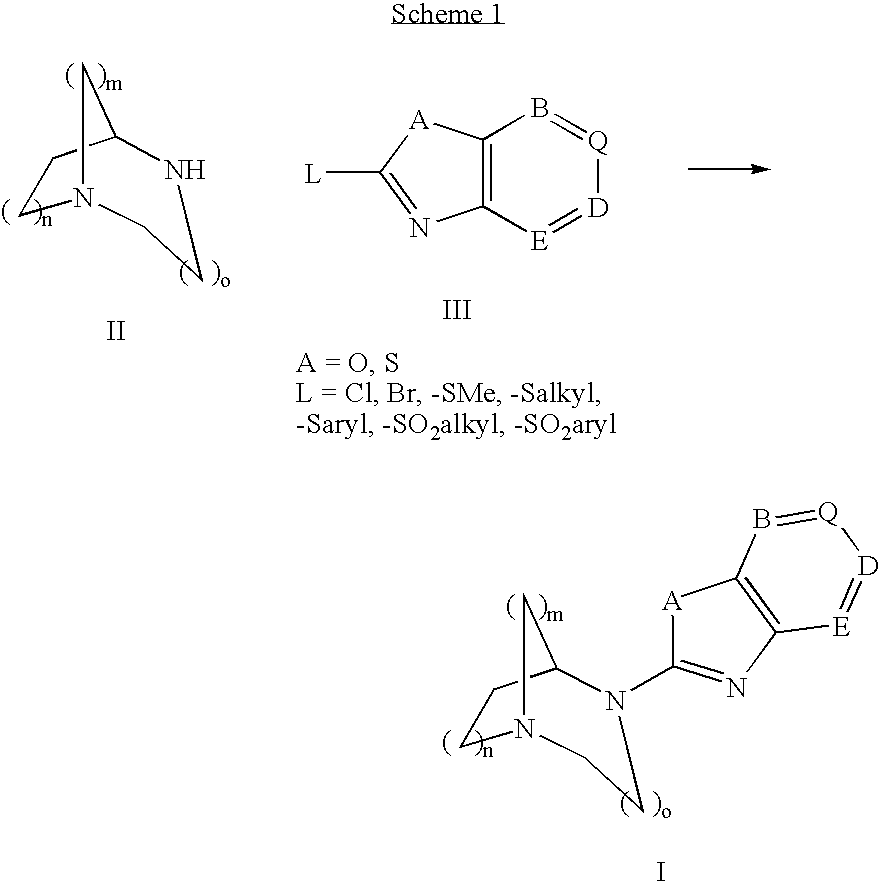
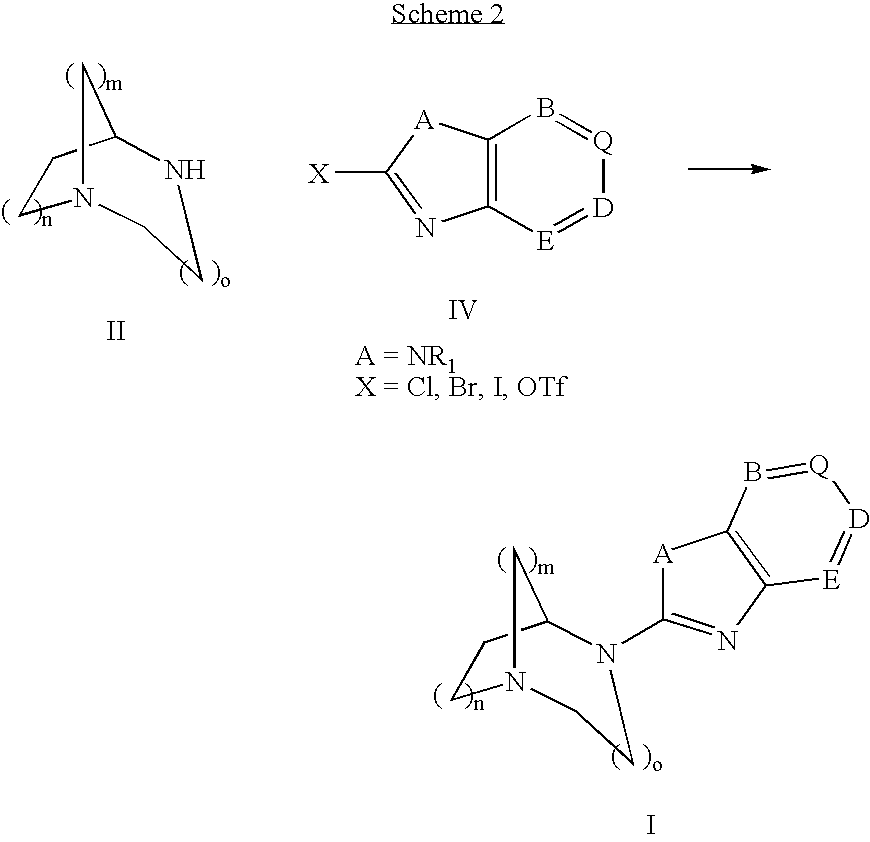
Pharmaceutical composition for the treatment of CNS and other disorders
a technology of cns and compositions, applied in the field of central nervous system disorders, can solve the problems of difficult control of anxiety and worry, difficulty in concentration, etc., and achieve the effects of improving the half-life of in vivo, facilitating preparation and detection, and reducing the risk of adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
4-BENZOOXAZOL-2-YL-1,4-DIAZA-BICYCLO[3.2.2]NONANE
[0126] 2-Chlorobenzoxazole (Aldrich, 99 μL, 0.87 mmol) was added to a solution of 1,4-diazabicyclo[3.2.2]nonane (100 mg, 0.79 mmol) in methanol (2.65 mL) at 0° C. The reaction mixture was allowed to slowly warm to RT. After a period of 16 h iPr2NEt (138 μL, 0.79 mmol) was added and the mixture was stirred at RT for 4.5 h at which time it was diluted with CHCl3 and NaHCO3. The layers were partitioned and the aqueous layer was extracted with CHCl3 (×3). The combined organic layers were washed with H2O and brine, dried (Na2SO4), filtered and concentrated. The crude residue was purified by chromatography (Biotage, 12L) eluting with 4% MeOH in CHCl3 containing 20 drops of NH4OH per liter of eluent to afford 67 mg (35%) of the title compound as a yellow oil: 1H NMR (CDCl3, 400 MHz) δ 7.30 (d, 1H, J=7.5 Hz), 7.19 (d, 1H, J=7.9 Hz), 7.10 (t, 1H, J=7.5 Hz), 6.94 (t, 1H, J=7.9 Hz), 4.46, (s, 1H), 3.87, (t, 2H, J=5.8 Hz), 3.12-2.92 (m, 6H), 2.1...
example 2
4-BENZOTHIAZOL-2-YL-1,4-DIAZA-BICYCLO[3.2.2]NONANE
[0127] 2-Chlorobenzothiazole (Aldrich, 109 μL, 0.841 mmol) was added to a solution of 1,4-diazabicyclo[3.2.2]nonane (57%, 169 mg, 0.765 mmol), Et3N (213 μL, 1.53 mmol) in DMF (2.5 mL). The reaction mixture was heated at 100° C. for 2 h. The mixture was allowed to cool to RT, diluted with EtOAc and H2O and the layers were partitioned. The aqueous layer was extracted with EtOAc (3×) and the combined organic extracts were washed successively with H2O and brine and then dried (Na2SO4), filtered and concentrated. The crude residue was purified by chromatography (Biotage, 12L) eluting with 5% MeOH in CHCl3 to afford 68 mg (34%) of the title compound as a yellow oil: 1H NMR (CDCl3, 400 MHz) δ 7.58 (d, 1H, J=7.9 Hz), 7.57 (d, 1H, J=7.9 Hz), 7.27 (t, 1H, J=8.3 Hz), 7.04 (td, 1H, J=7.9, 1.2 Hz), 4.31 (s, 1H), 3.92 (t, 2H, J=5.8 Hz), 3.19-2.98 (m, 6H), 2.25-2.16 (m, 2H), 1.84-1.77 (m, 2H); MS (Cl) m / z 260.2 (M+1). The hydrochloride salt was pr...
example 3
5-PHENYL-3H-BENZOOXAZOLE-2-THIONE
[0129] Carbon disulfide (7.7 mL) was added to a mixture of 2-amino-4-phenylphenol (1.0 g, 5.4 mmol), potassium hydroxide. (0.36 g, 6.5 mmol) and ethanol (11.7 mL). The flask was fitted with a reflux condenser and the resulting mixture was placed in an oil bath at 60° C. for 16 h. After cooling to RT, the mixture was concentrated and ethyl acetate (20 mL) and 1 M hydrochloric acid (10 mL) were added to the residue. The layers were partitioned and the organic layer was washed successively with 1 M HCl, water and brine. The organic layer was dried (Na2SO4), filtered and concentrated to afford 1.20 g (98%) which was used without further purification: 1H NMR (d6-DMSO, 400 MHz) δ 13.98 (s, 1H), 7.64-7.62 (m, 2H), 7.58-7.49 (m, 2H), 7.46-7.42 (m, 2H), 7.39-7.33 (m, 2H); 13C (d6-DMSO, 400 MHz) δ 181.2, 148.4, 140.1, 138.5, 132.7, 129.7, 128.3, 127.7, 123.4, 111.0, 109.1; MS (Cl) m / z 228.1 (M+1); HPLC retention time=3.09 min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com