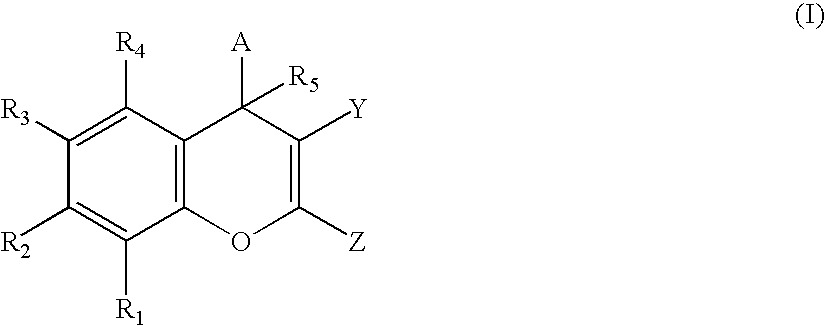
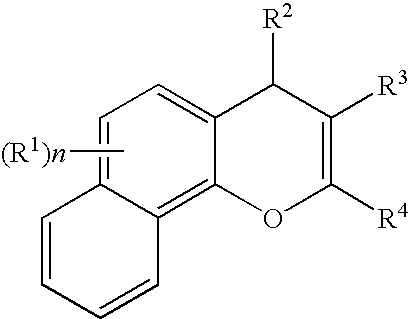
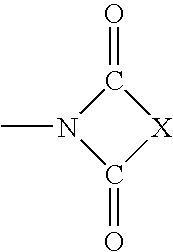
Substituted 4h-chromenes and analogs as activators of caspases and inducers of apoptosis and the use thereof
a caspase activator and analog technology, applied in the field of substituted 4hchromenes and analogs, can solve problems such as bone marrow toxicity, and achieve the effect of treating, preventing or ameliorating neoplasia and cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
2-Amino-3-cyano-7-hydroxy-4-(3-bromo-4,5-dimethoxyphenyl)-4H-chromene
[0369] To a mixture of 3,4-dimethoxy-5-bromobenzylidenemalononitrile (293 mg, 1 mmol) and resorcinol (110 mg, 1 mmol) in ethanol (2 mL) was added piperidine (0.1 mL, 1 mmol). The mixture was refluxed for 2 h. The solvent was evaporated, the residue was purified by chromatography on silica gel with EtOAc and hexane (1:2) as eluant, yielding 240 mg (59.5%) of the title compound. 1H NMR (DMSO-d6): 9.77 (brs, 1H), 6.96-6.86 (m, 5H), 6.52 (d, J=8.1, 1H), 6.41 (s, 1H), 4.65 (s, 1H), 3.80 (s, 3H), 3.70(s, 3H).
example 2a
2-Amino-3-cyano-7-ethylamino-4-(3-bromo-4,5-dimethoxyphenyl)-4H-chromene
[0370] To a mixture of 5-bromoveratraldehyde (245 mg, 1 mmol) and malononitrile (66 mg, 1 mmol) in ethanol (2 mL) was added piperidine (0.1 ml, 1 mmol) and 3-ethylaminephenol (140 mg, 1 mmol). The mixture was stirred at room temperature overnight. The solvent was evaporated, the residue was purified by chromatography on silica gel with EtOAc and hexane (1:2) as eluant, yielding (330 mg, 76.7%) title compound. 1H NMR (CDCl3): 6.88 (d, J=0.9 Hz, 1H), 6.71 (d, J=8.4 Hz, 2H), 6.32 (dd, J=2.1 Hz, 1H), 6.19 (d, J=2.1 Hz, 1H), 4.59 (s, 2H), 4.54 (s, 1H), 3.83 (d, J=0.6 Hz, 3H), 3.82 (d, J=0.9 Hz, 1H), 3.68 (brs, 1H), 3.12 (q, J=7.2 Hz, 2H), 1.28-1.23 (m, 3H).
[0371] The following compounds were prepared by a procedure similar to that described in Example 2A.
example 2b
2-Amino-3-cyano-7-hydroxy-4-(3-cyanophenyl)-4H-chromene
[0372]1H NMR (DMSO-d6): 7.70-7.65 (m, 2H), 7.55-7.47 (m, 2H), 6.99 (brs, 2H), 6.79 (d, J=8.9 Hz, 1H), 6.48 (dd, J=2.5, 8.4 Hz, 1H), 6.40 (d, J=2.5 Hz, 1H), 4.76 (s, 1H) ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap