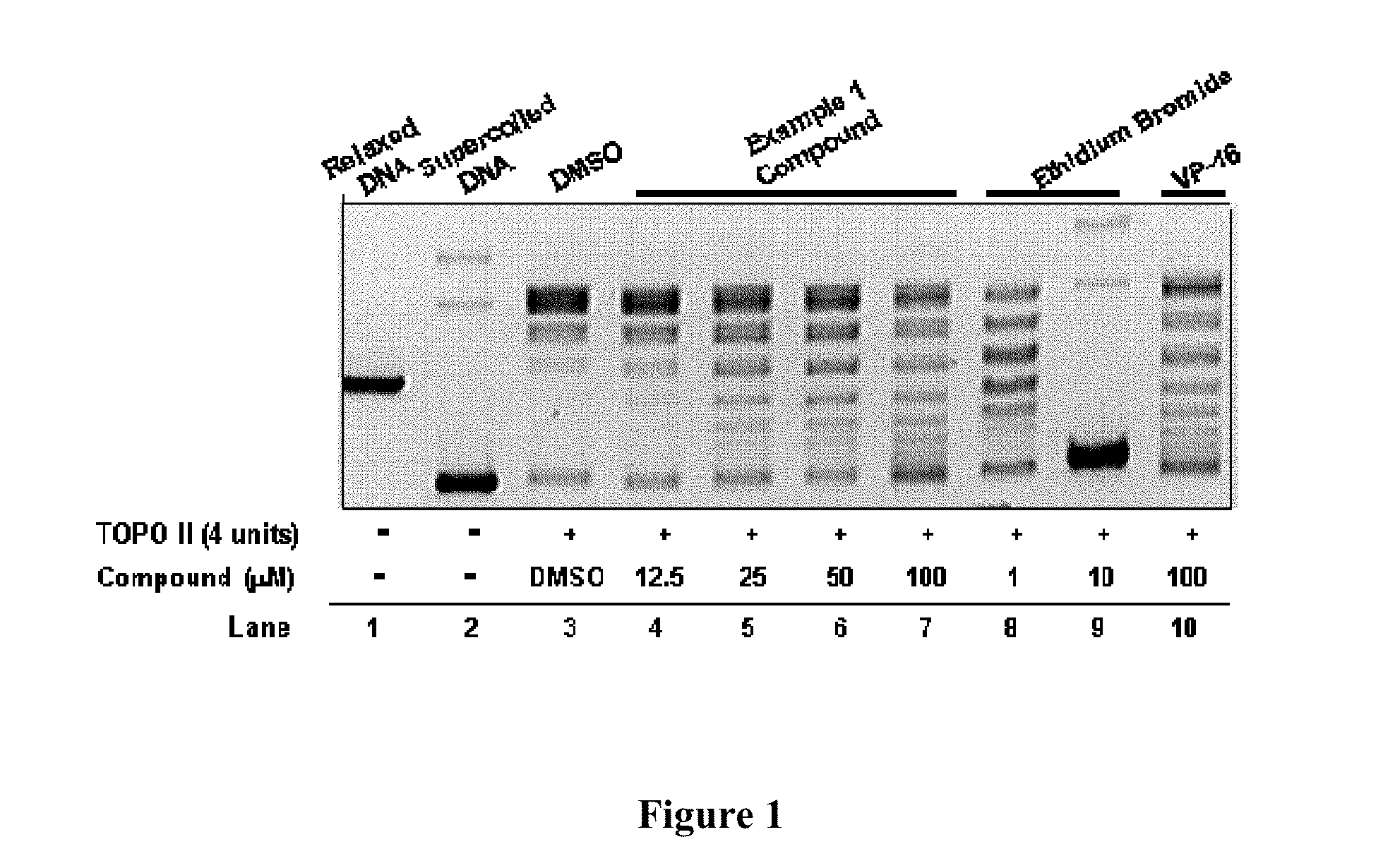
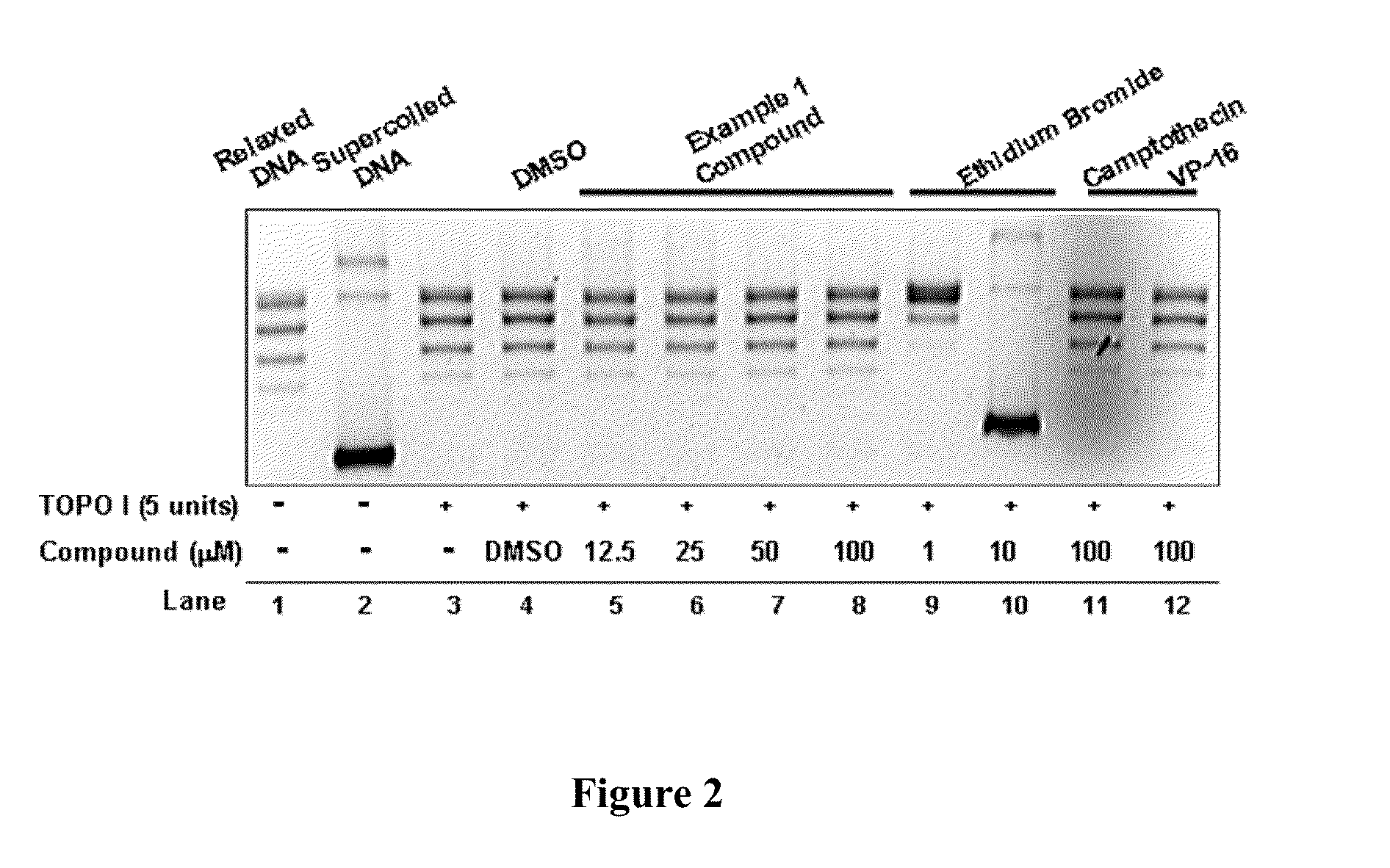
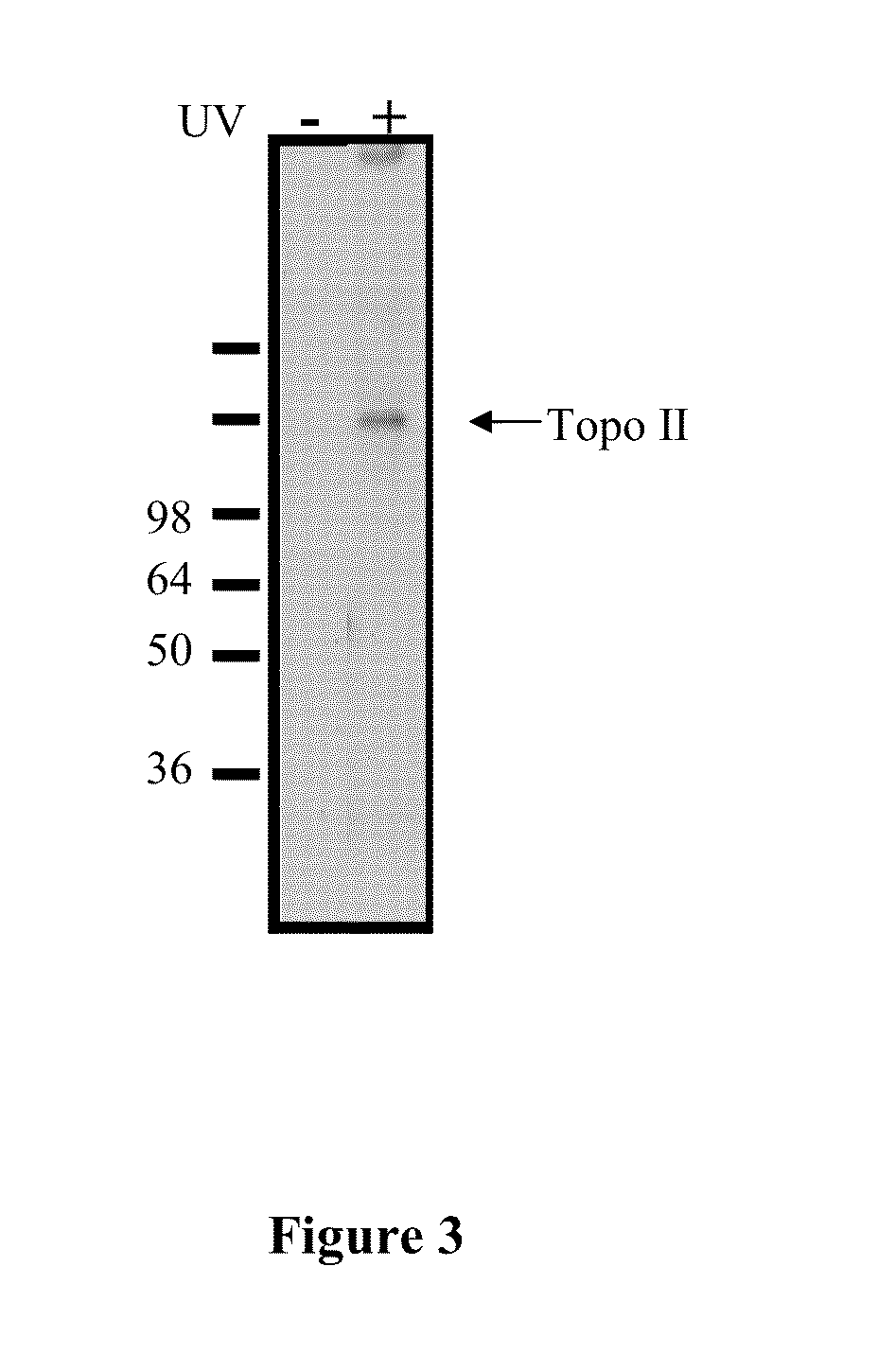
Compounds and therapeutical uses thereof
a technology of compounds and active ingredients, applied in the field of medicinal chemistry, can solve the problems of bone marrow toxicity, cancer cells losing the capacity to undergo cell suicide, and toxicity of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[1233]
(2-Chloro-quinazolin-4-yl)-(4-methoxy-phenyl)-methyl-amine
[1234]a) 2,4-Dichloroquinazoline: A suspension of 2,4-quinazolinedione (5.0 g, 30.8 mmol) in neat phosphorylchloride (50 mL) was heated under reflux for 18 h. The reaction mixture was concentrated under vacuum. The crude product was purified by chromatography (Silica gel) using ethyl acetate and hexane (1:4) to give 2,4-dichloroquinazoline as white solid (4.8 g, 96%). 1H NMR (CDCl3): 8.29 (ddd, J=8.4, 2.1 and 0.9 Hz, 1H), 8.04-8.00 (m, 2H), 7.75 (ddd, J=8.1, 4.8 and 3.0 Hz, 1H).
[1235]b) (2-Chloro-quinazolin-4-yl)-(4-methoxy-phenyl)-methyl-amine: A solution of 2,4-dichloroquinazoline (300 mg, 1.51 mmol) and 4-methoxy-N-methylaniline (248 mg, 1.81 mmol) in 5 ml isopropanol with a drop of concentrated HCl was stirred at room temperature for 8 h. White precipitates were observed in the reaction mixture. The reaction was filtered, and the solid was washed with isopropanol, and dried under vacuum to give white powder (260 mg,...
example 2
[1236]
(2-Chloro-quinazolin-4-yl)-(4-methyl-phenyl)-methyl-amine
[1237]The title compound was prepared from 2,4-dichloroquinazoline (250 mg, 1.25 mmol) and 4-methyl-N-methylaniline (196 mg, 1.43 mmol) by a procedure similar to Example 1b and was isolated as white powder (210 mg, 84%). 1H NMR (CDCl3): 8.69 (d, J=8.4 Hz, 1H), 7.75 (dd, J=8.1 and 7.5 Hz, 1H), 7.39 (d, J=7.8 Hz, 2H), 7.25 (d, J=7.8 Hz, 2H), 7.13 (d, J=8.2 Hz, 1H), 6.74 (d, J=8.7 Hz, 1H), 3.81 (s, 3H), 2.49 (s, 3H).
example 3
[1238]
(2-Chloro-quinazolin-4-yl)-(4-chloro-phenyl)-methyl-amine
[1239]The title compound was prepared from 2,4-dichloroquinazoline (60 mg, 0.302 mmol) and 4-chloro-N-methylaniline (50 mg, 0.332 mmol) by a procedure similar to Example 1b and was isolated as white powder (30 mg, 50%). 1H NMR (CDCl3): 8.66 (d, J=8.4 Hz, 1H), 7.78 (ddd, J=8.1, 7.5 and 2.4 Hz, 1H), 7.57 (d, J=8.7 Hz, 2H), 7.28 (d, J=8.7 Hz, 2H), 7.19 (ddd, J=8.1, 7.5 and 2.4 Hz, 1H), 6.79 (d, J=8.4 Hz, 1H), 3.83 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com