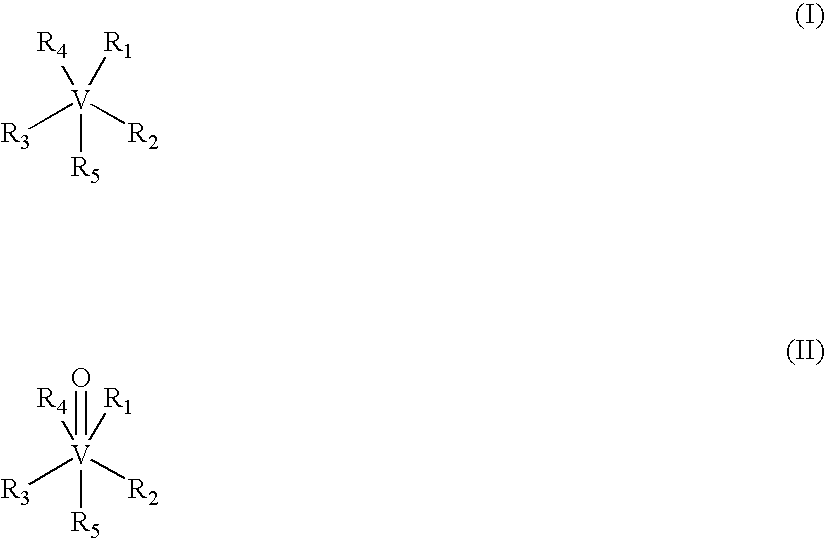Vanadium compounds as anti-angiogenic agents
a technology of vanadium compounds and anti-angiogenic agents, which is applied in the direction of biocide, heavy metal active ingredients, drug compositions, etc., can solve the problem of 30%-50% incidence of coronary artery restenosis, and achieve the effect of inhibiting angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Vanadium Compounds
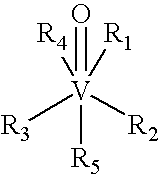
[0100] Vanadium compounds useful in the invention may be prepared by known methods, as described, for example, in published PCT Applications WO99 / 36063; WO 00 / 27389; and WO 00 / 35930. For example, VDC (VCp2Cl2) and. [VCp2(acac)](CF3SO3); (VDacac) were prepared by following literature procedures (Wilkinson et al., J. Am. Chem. Soc., 76: 4281-4284, 1954; Doyle et al., Inorg. Chem., 7: 2479-2484, 1968) and purity was confirmed by 1H NMR, IR spectroscopy, and elemental analysis.
example 2
Inhibition of Angiogenesis
[0101] The present example illustrates that vanadium compounds such as [VCp2(acac)](CF3SO3) are effective inhibitors of angiogenesis.
Chick Embryo Chorioallantoic (CAM) Assay
[0102] Inhibition of embryonic angiogenesis was determined using a bioassay system involving CAMs of growing chick embryos, as previously described (Nguyen et al, Microvascular Research, 47:31, 1994; Auerbach et al, Developmental Biology, 41:391, 1974). Fertilized white Leghorn chicken eggs were received at day 3 of incubation from the University of Minnesota Poultry Research Center, St. Paul, Minn. The following procedures took place in a sterile laminar flow hood. The eggs were wiped down with 70% isoprpyl alcohol and allowed to air dry. The eggs were wiped with Betadine and placed in a horizontal position for approximately 5 minutes and allowed to dry. The eggs were cracked and placed into sterile 100×20 mm2 Petri dishes (Fischer, Itasca, Ill.) and transferred to a 37° C. humidifi...
example 3
Inhibition of Mitosis
[0106] This example illustrates that the vanadium compound [VCp2(acac)](CF3SO3) is an effective inhibitor of embryonic development of Zebra fish, and in particular, is a potent inhibitor of mitosis.
Zebra Fish and Embryos
[0107] The adult wild type ZF were maintained generally according to the “Zebrafish Book” recommendations (Westerfield, The Zebrafish Book, 1993, 2d Edition Univ. of Oregon Press, Eugene). Males and females were kept in 10 Gallon tanks (70 fish per tank) with a constant slow flow of conditioned water at 26° C. and a controlled 14 hour day / 10 hour night cycle. Adult fish were fed twice a day with live brine shrimp (Ocean Star International, Snowville, Utah) and each group of fish was bred once in two weeks. The embryos were obtained through (a) natural spawning at 28.5° C. in the breeding tanks with a netted false bottom or (b) fertilization in vitro using eggs and milt collected from the mature females and males anesthetized with Tricaine (Si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| inner path length | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com