Non-pegylated liposomal doxorubicin triple combination therapy
a combination therapy and non-pegylated technology, applied in the field of metastatic breast cancer treatment, can solve the problems of limited use of doxorubicin, patients suffering irreversible cardiotoxicity, and serious side effects of doxorubicin and sometimes life-threatening side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Single Use Vial
[0048]Vial #1 Doxorubicin HCl for Injection is provided in glass vials sealed with butyl rubber stoppers and aluminum flip-off seals which contains:
Doxorubicin HCl, USP 50 mgLactose, NF (hydrous)250 mg
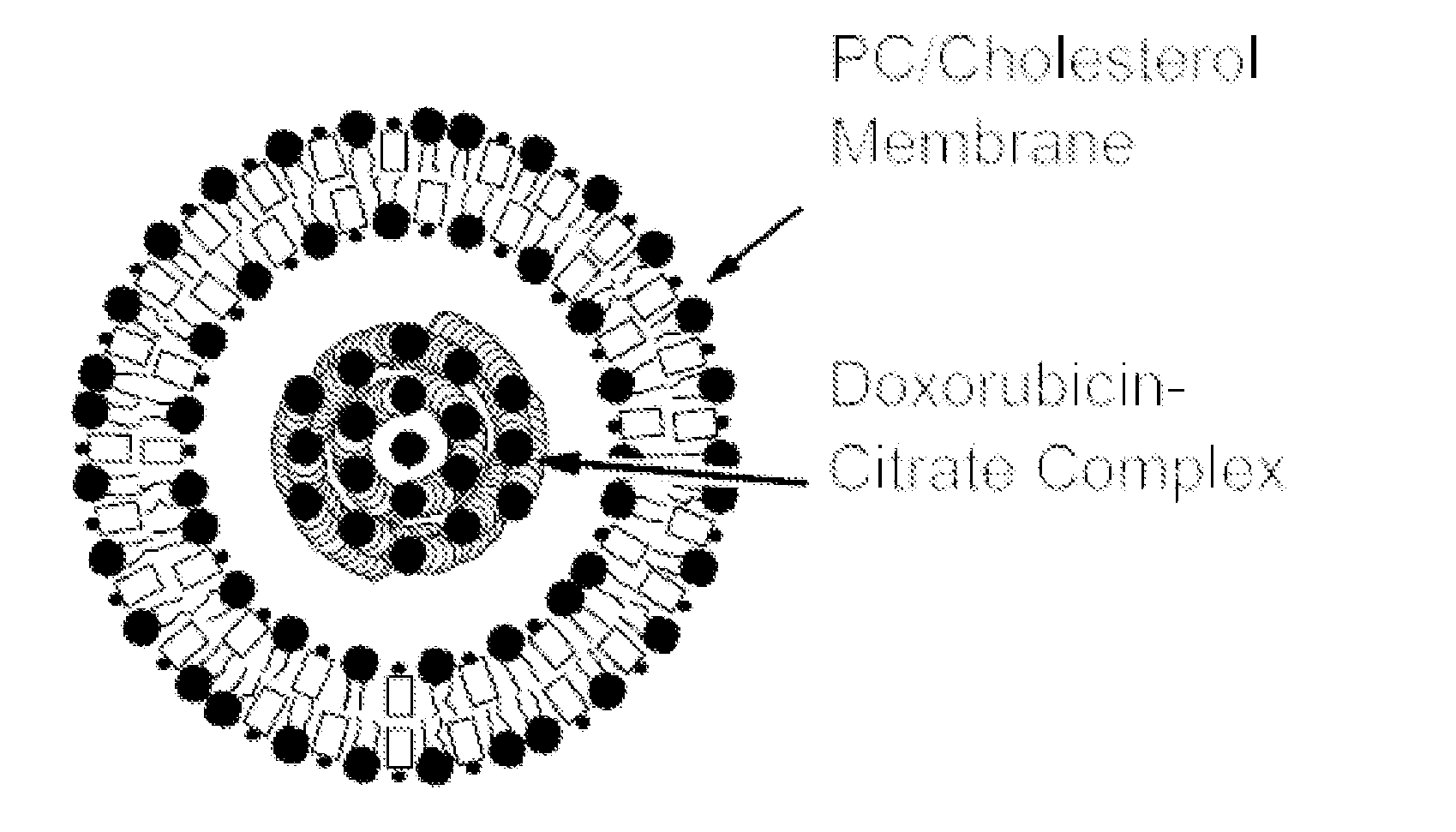
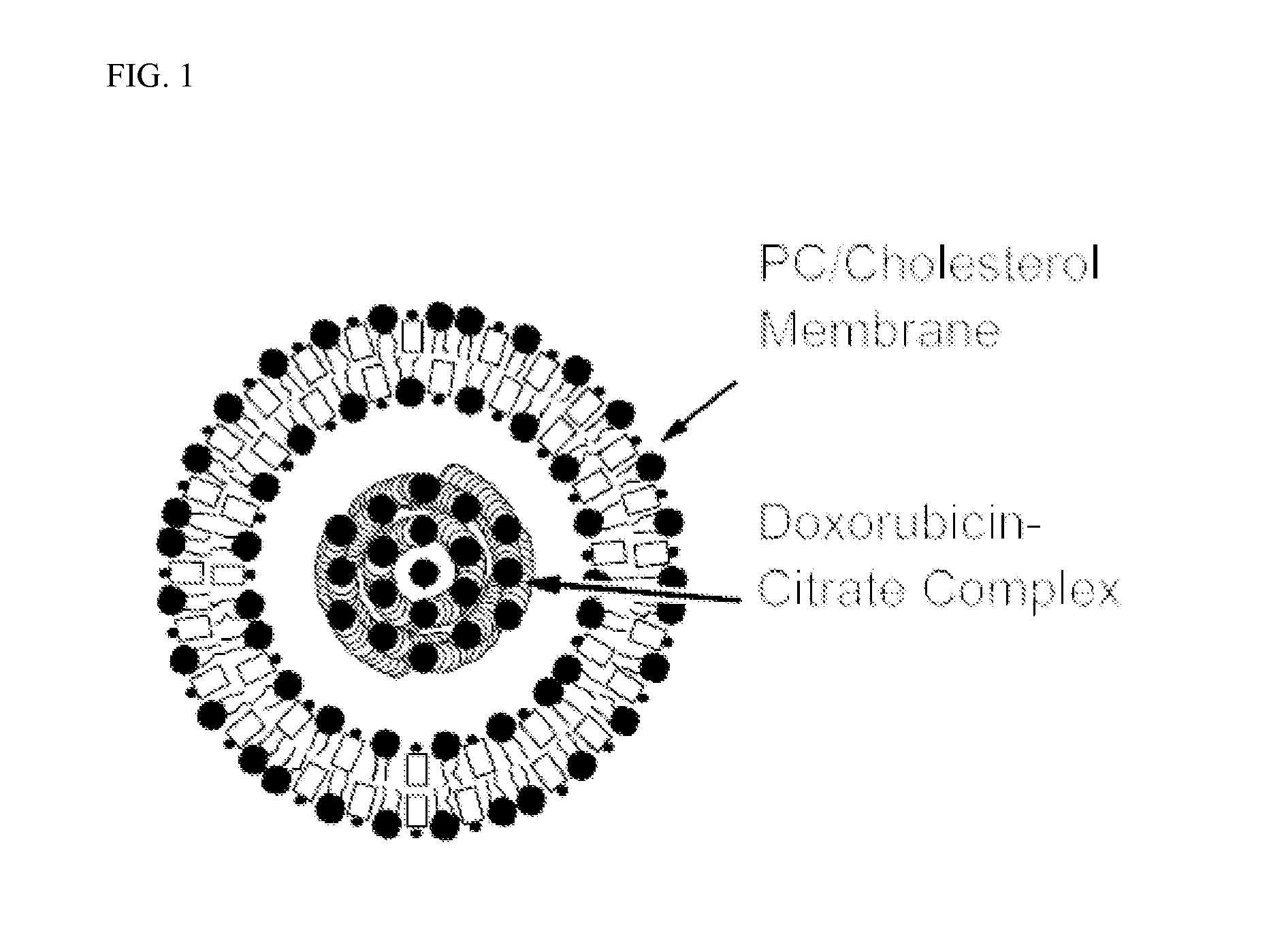
[0049]Vial #2 Liposomes for Injection is provided in 2 ml type I flint glass tubing vial with grey stoppers siliconized with dimethicone and a flip-off seal which contains:
Egg Phosphatidylcholine142.6 mgCholesterol, NF 57.4 mgCitrate Buffer (57.6 mg / mL) q.s. 2 mL
[0050]Vial #3 Buffer for Injection is provided in 5 ml type I molded glass vials with grey stoppers siliconized with dimethicone and a flip-off seal contains:
Sodium Carbonate anhydrous, NF54.6 mgWater for Injection, USP q.s. 3.1 mL
[0051]Each prepared vial of nonpegylated liposomal doxorubicin contains 50 mg of doxorubicin HCl, and each milliliter of nonpegylated liposomal doxorubicin contains:
Doxorubicin HCl 2.0 mgEgg phosphatidylcholine 5.4 mgCholesterol 2.2 mgCitric acid, monohydrate 4.4 mgSodi...
example 2
[0075]The following study is being conducted to evaluate the safety and efficacy of the combination of non-pegylated liposomal doxorubicin (MYOCET®), paclitaxel and trastuzumab as first-line treatment in patients with HER2-over-expressing metastatic breast cancer. Inclusion criteria for the study are: (1) metastatic HER2+ breast cancer by FISH analysis; (2) no prior chemotherapy for metastatic disease; (3) measurable disease; and (4) normal left ventricular ejection fraction. Exclusion criteria are: (1) prior doxorubicin treatment exceeding 300 mg / m2 or prior epirubicin treatment exceeding 600 mg / m2; and (2) relapse within 12 months of completion of adjuvant trastuzumab, taxane or anthracycline therapy.
[0076]The primary objectives of this trial are to demonstrate the efficacy and cardiac safety of Myocet® when given in combination with trastuzumab and paclitaxel in patients with HER2+ metastatic breast cancer.
[0077]Within the population of patients who are administered a dosing regi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean diameter | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| swelling | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com