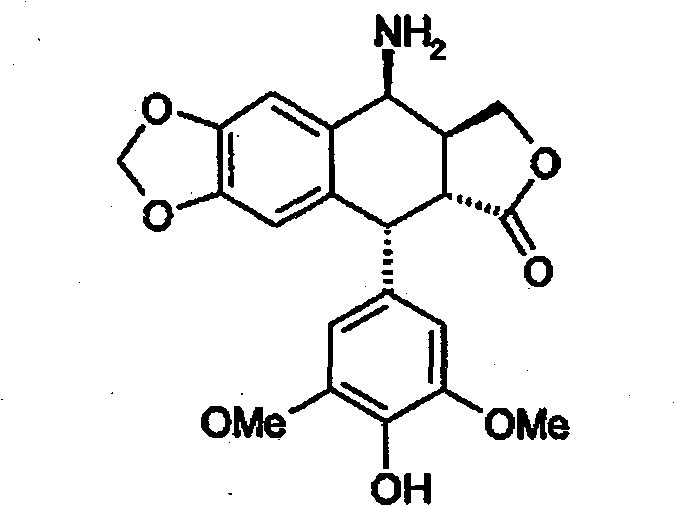
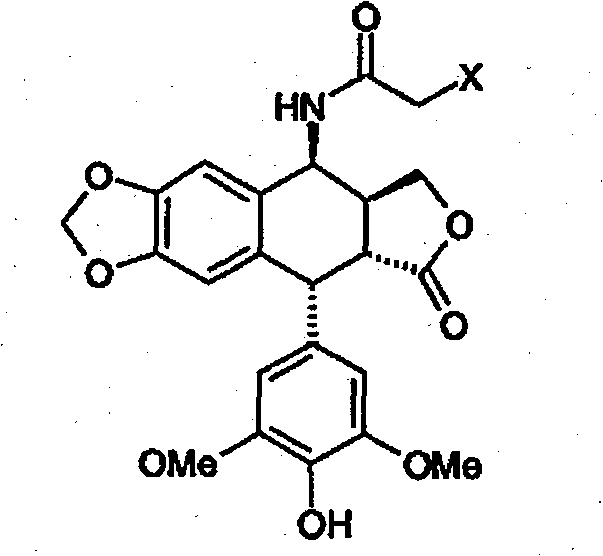
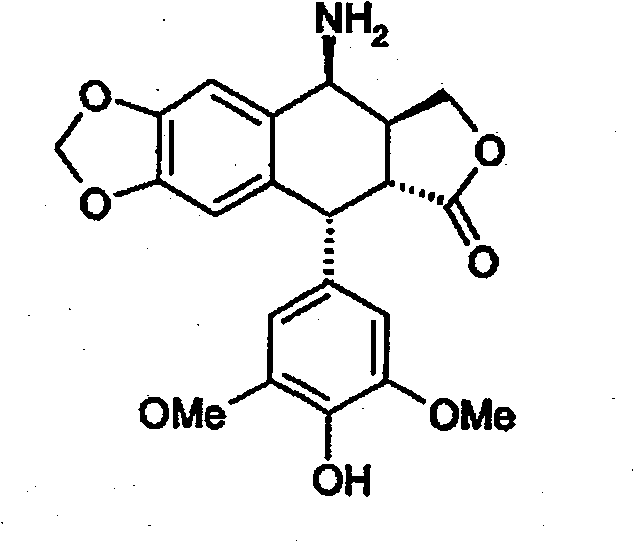
Method for preparing 4beta,-amino-4'-demethyl-4-desoxypodophyllotoxin
A technology of deoxypodophyllotoxin and demethylated epipodophyllotoxin, which is applied in organic chemistry and other fields, and can solve problems such as unfavorable reaction time and occurrence of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 : Preparation of 4β-chloroacetamido-4'-desmethyl-4-deoxypodophyllotoxin (Formula 3).
[0065] 0.5 mL of concentrated sulfuric acid was added dropwise to a suspension of 30 g (0.075 mol) of 4'-desmethylepipodophyllotoxin in 47.5 mL (0.75 mol) of chloroacetonitrile at ambient temperature. It was stirred at this temperature for 1 h, dissolved, and reprecipitation was observed. Add 300 mL of 2-propanol. The precipitate was filtered and rinsed with 200 mL of 2-propanol and water to return to pH 7. The resulting white solid was dried under vacuum at 40°C to afford 32.9 g of the chloroacetamido compound of formula 3, or a molar yield of 93%.
[0066] Melting point F=240°C.
[0067] NMR analysis of protons: 1 H RMN(DMSO)δ8,65(d,1H,J=7Hz,NH),8,26(s,1H,4'-OH),6,78(s,1H,H 5 ), 6,54(s, 1H, H 8 ), 6, 24(s, 2H, H 2′ , H 6′ ), 5,99 (d, 2H, J=11.3Hz, OCH 2 O), 5, 17 (dd, 1H, J=4, 56 and 7Hz, H 4 ), 4,51 (d, 1H, J=5,2Hz, H 1 ), 4, 29 (t, 1H, J=8Hz, H 11a ), 4...
Embodiment 2
[0072] Example 2 : Preparation of 4β-amino-4'-desmethyl-4-deoxypodophyllotoxin (formula 1) - method using pure glacial acetic acid - first method according to the invention
[0073] Table 1: No. 2.
[0074] A suspension of 17 g (0.0358 mol) of 4β-chloroacetamido-4'-demethyl-4-deoxypodophyllotoxin obtained in Example 1 in 75 mL of glacial acetic acid was heated to 80° C. under stirring. Add 4.2 g (0.0537 mol) thiourea in one portion. It was stirred at this temperature for 1 hour and 30 minutes, dissolved, and reprecipitation was observed. The reaction medium was filtered hot and rinsed with 75 mL of glacial acetic acid and diisopropyl ether. The resulting white solid was dried under vacuum at 40° C. to afford 14.6 g of the compound of formula 1 as the hydrochloride salt, corresponding to a molar yield of 93%.
[0075] Melting point F>260°C.
[0076] NMR analysis of protons: 1 H RMN(DMSO)δ8,63(m,2H),8,32(m,1H),7,23(s,1H,H 5 ), 6,60(s, 1H, H 8 ), 6, 18(s, 2H, H 2′ , H ...
Embodiment 3
[0077] Example 3 : Preparation of 4β-amino-4'-desmethyl-4-deoxypodophyllotoxin (formula 1) - method using ethanol and 1N hydrochloric acid - first possibility of the first variant of the second method according to the invention alternative plan.
[0078] Table 1: No. 3.
[0079] A suspension of 0.5 g (1.05 mmol) of 4β-chloroacetamido-4'-demethyl-4-deoxypodophyllotoxin obtained in Example 1 in a mixture of 2.5 mL of ethanol and 1 mL of 1N hydrochloric acid was The temperature was raised to 80°C with stirring. 0.12 g (1.57 mmol) thiourea was added in one portion. It was stirred at this temperature for 9 hours, dissolved, and reprecipitation was observed. The cooled reaction medium is filtered, rinsed with ethanol and diisopropyl ether. The resulting white solid was dried under vacuum at 40° C. to afford 0.4 g of the compound of formula 1 as the hydrochloride salt, corresponding to a molar yield of 90%.
[0080] Melting point F>260°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com