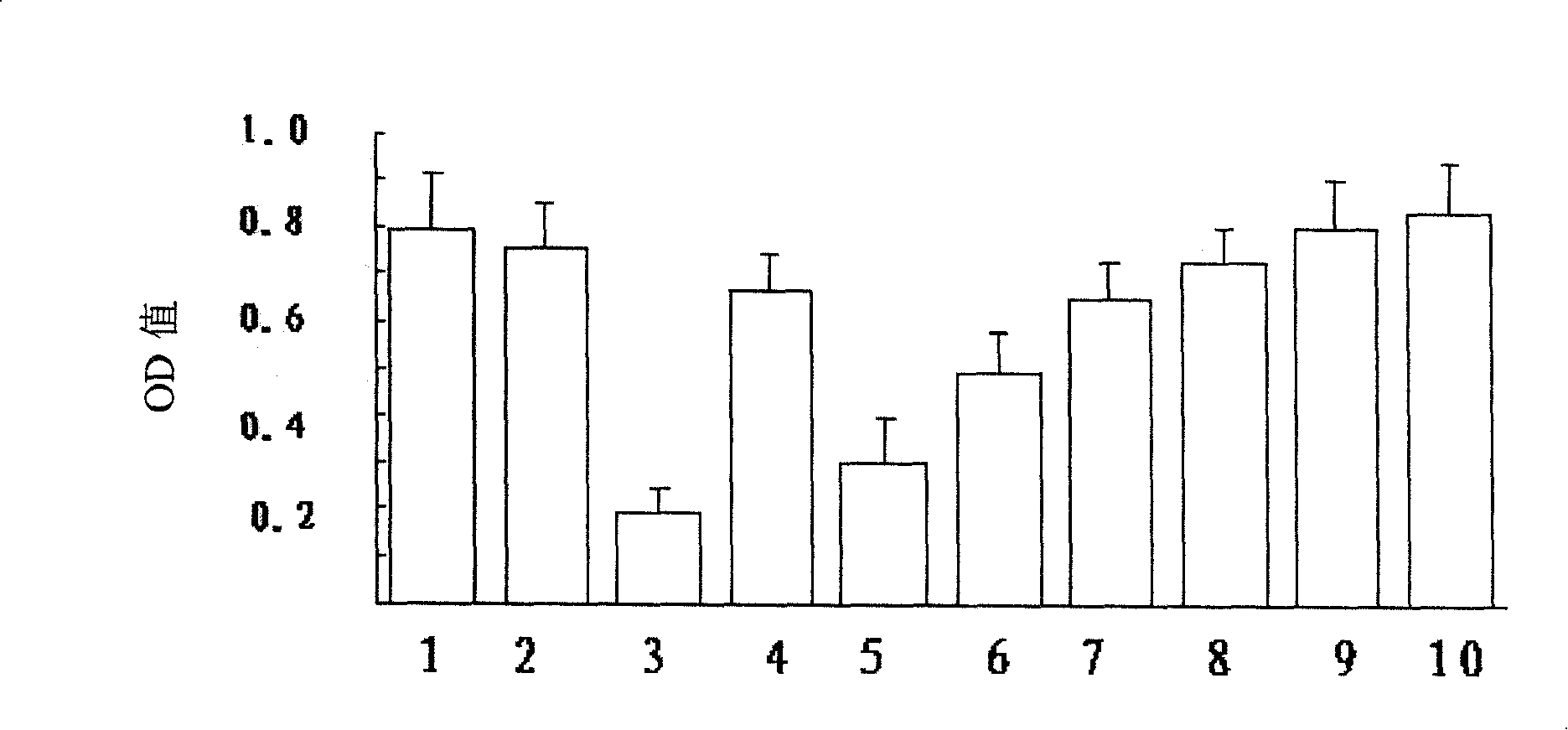Application of dual inhibitor for dopamine and norepinephrine transfer protein
A technology of norepinephrine and dual inhibitors, applied in the preparation of drugs for the treatment of central nervous system degenerative diseases, in the field of dual inhibitors of dopamine transporter and norepinephrine transporter, which can solve the problem of accelerating dopaminergic nerve cells , changing the course of the disease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1, establishment of in vitro screening model
[0095] Establishment of activity screening cell lines targeting serotonin, norepinephrine, dopamine, and γ-aminobutyric acid four neurotransporters (5-HTT, NET, DAT, GAT-1). By the rat 5-HTT, NET, DAT, GAT-1 full-length coding sequence (the GenBank accession number of 5-HTT is GI207086; the GenBank accession number of NET is GI4630791; the GenBank accession number of DAT is GI310097; GAT-1 The GenBank accession number is GI204221) cloned in the multiple cloning site of the pCDNA3 vector (Invitrogen, USA), transfected into Chinese hamster ovary cells (CHO) by electroporation, and cultured in 1640 medium containing G418 after 48 hours. After 10 days, all the cells in the control group died, while many cell clones formed in the experimental group. After the clones were picked and cultured for one week, when the cells covered the bottom of the well, the medium was sucked off, and digested with trypsin as above. Cel...
Embodiment 2
[0096] Example 2. In vitro screening of dopamine transporter agonists and determination of selection specificity
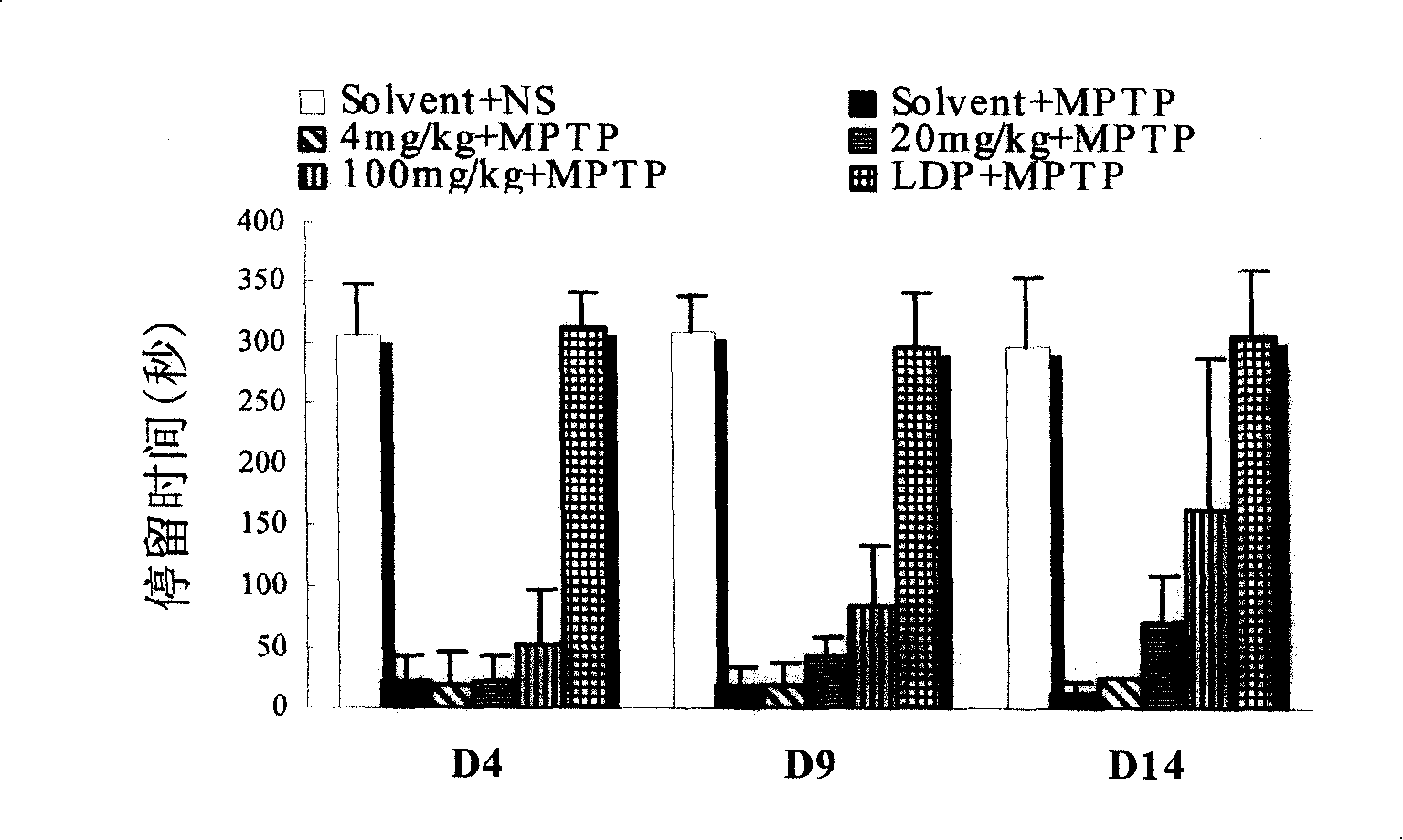
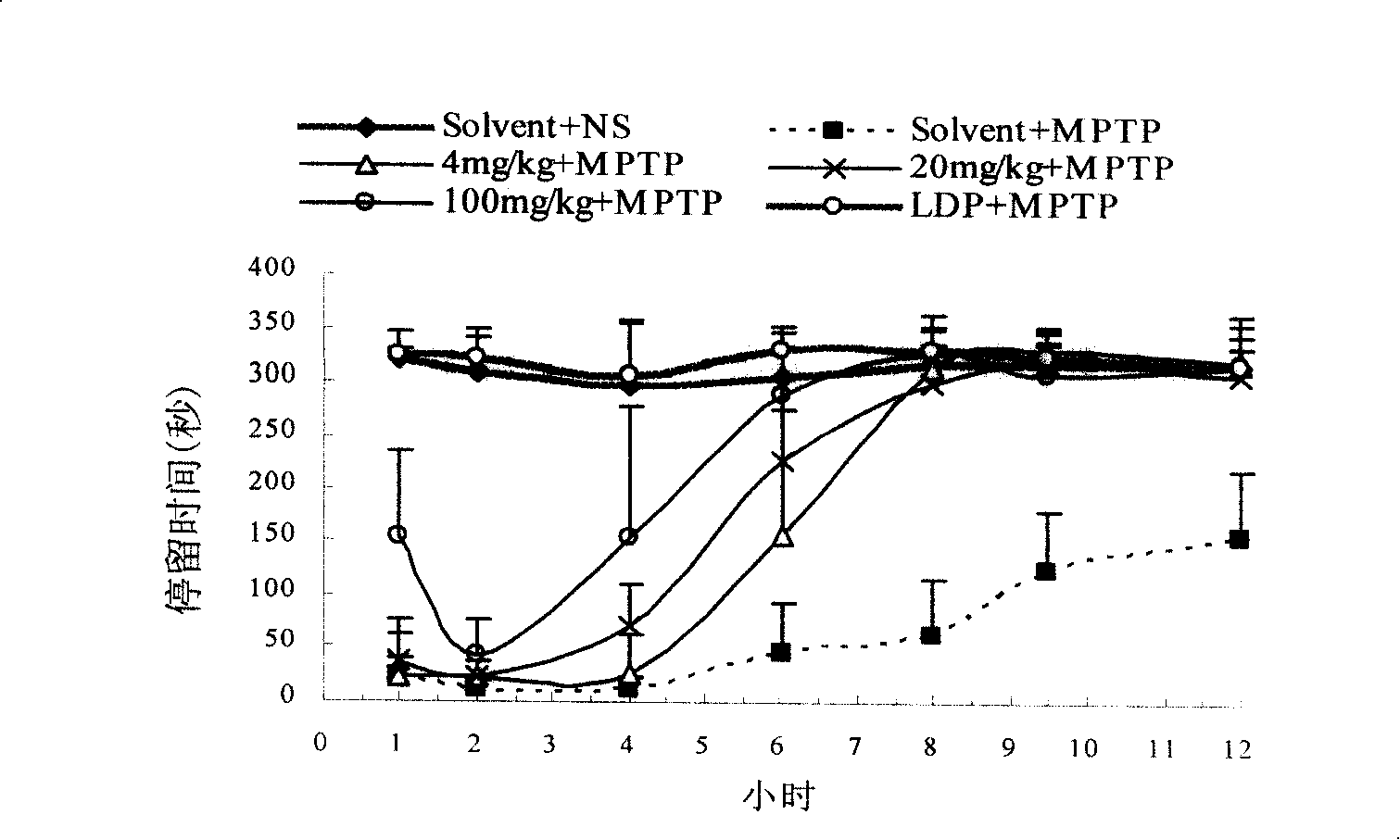
[0097] D8 cells were cultured in a 48-well plate (Costar) until the plate was confluent (approximately 60,000 cells per well). Discard culture medium. Wash once with PBS, suck off the PBS solution, add 90ul HBS (10mM Hepes, 100mM NaCl, pH8.0) to each well, incubate at 25°C for 10 minutes, and add 10ul HBS reaction solution to each well. Add 80ul HBS, 10ul drugs of different concentrations, and 10ul 3 H-DA (Amersham Pharmacia Biotech), 100 μM vitamin C and 100 μM parjiline. Incubate at 25°C for 20 minutes, wash three times with ice-bathed PBS solution, lyse with lysate for 60 minutes, absorb the lysate from each well, add it to 1.2ml scintillation fluid, and put it into a liquid scintillation counter (Beckman LS 5000TA) The content of the isotope (DMP value) is detected in the drug to measure the influence of the drug on the transport activity of the dopamine tr...
Embodiment 3
[0108] Example 3 Cytotoxicity test of 13-hydroxyisobakuchiol and its inhibitory effect on MPP+ cytotoxicity
[0109] Cultivate D8 cells in 1640 medium, add 10% calf serum, and after the culture dish is full, digest with trypsin, inoculate in 48-well cell plate, and continue to cultivate until the number of cells reaches 10 5 Left and right were treated as follows: after changing the medium, add 10 μl of control solution (HBS containing 1% DMSO) to the control group, and add 10 μl of Bu with different concentrations to the different dosage groups of the drug, so that the final concentrations were 1 μM, 10 μM and 100 μM; , with 33 μM MPP + The cells were treated alone or together with different concentrations of Bu above; the cells were cultured for 24 hours, and MTT was added at the 20th hour to make the final concentration 0.5 mg / ml. After the reaction, the culture solution was sucked off, 100% DMSO was added, and incubated at 37° C. for 10 minutes. Transfer to a 96-well pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com