Highly selective serotonin and norepinephrine dual reuptake inhibitor and use thereof
A technology selected from, halogen, applied in the field of highly selective serotonin and norepinephrine dual reuptake inhibitors and their uses, which can solve the problems of high NE activity and moderate increase in diastolic blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0140] Example 1: Preparation of 1-[-2-dimethylamino-1-(4-phenol) ethyl]-cis-1,4-cyclohexanediol
[0141] with K 2 CO 3(35.6 g, 258.0 mmol) followed by benzyl bromide (28 mL, 238 mmol) in 4-(dimethylcarbamoylmethyl)phenol (35.6 g, 198.5 mmol). The mixture was stirred at room temperature for 4 days, then heated at 60 °C for 1 h. The mixture was concentrated to remove DMF, diluted with EtOAc and washed 3 times with water. Add anhydrous MgSO 4 , the mixture was filtered and concentrated to low volume. Hexane was added to precipitate the product. The solid was collected by filtration and dried to give 49 g, 92% yield of solid.
[0142]
[0143] A solution of 2N lithium diisopropylamide (LDA) (48.25 mL, 96.5 mmol) was cooled to -78 °C and diluted with 25 mL tetrahydrofuran (THF). To this was added dropwise a solution of 2-(4-benzyloxy-phenyl)-N,N-dimethyl-acetamide (20 g, 74.3 mmol) in 250 mL THF. The mixture was allowed to warm to 0°C, then cooled back to -78°C. A sol...
example 2
[0151] Example 2: Physical-chemical properties of 1-[-2-dimethylamino-1-(4-phenol)ethyl]-cis-1,4-cyclohexanediol
[0152] When prepared according to the method of Example 1, the characteristics of the title compound (free base) are as follows:
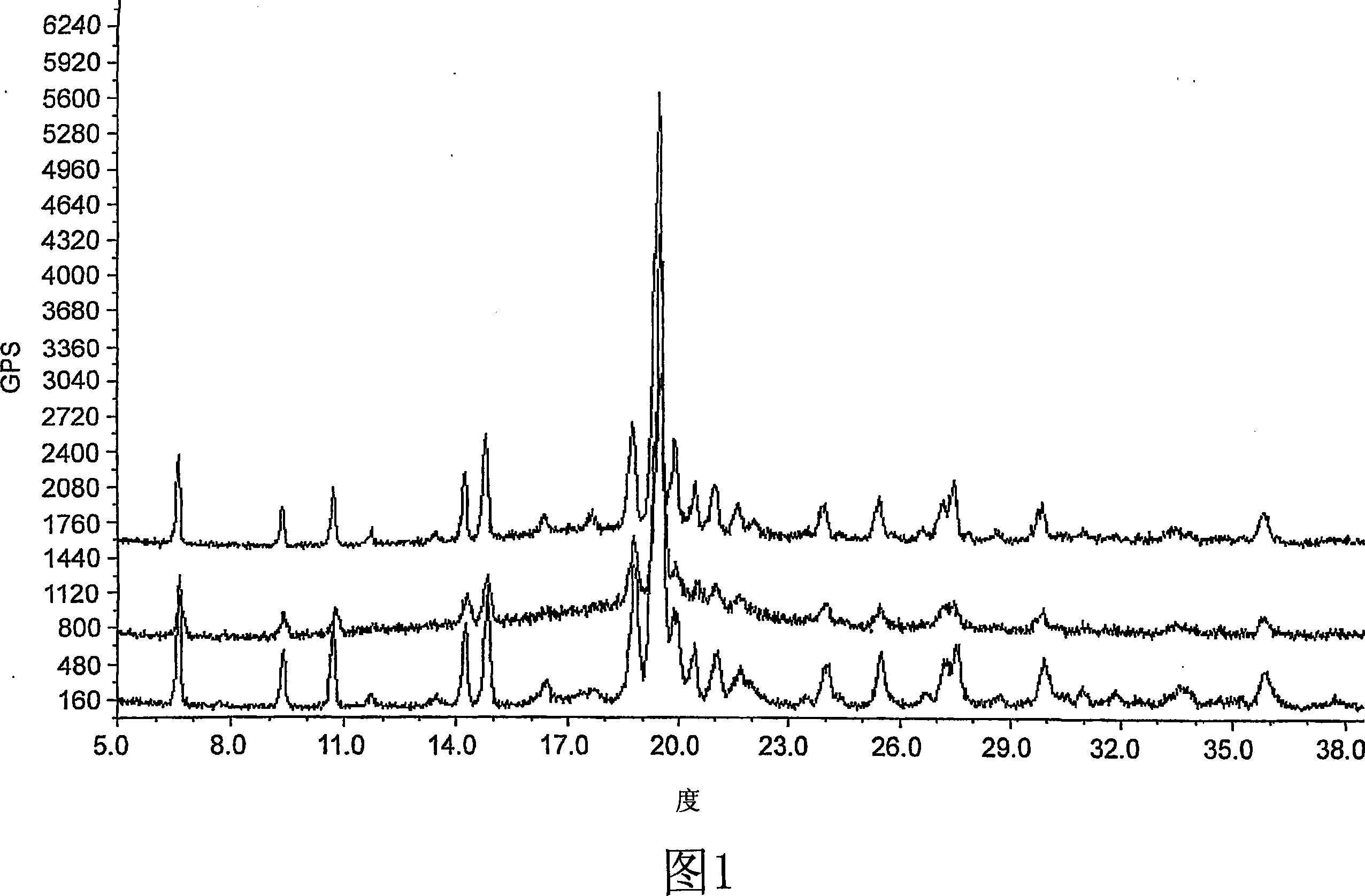
[0153] Purity 97.51% cis isomer, 1.91% trans isomer, 0.22% intermediate
[0154]
[0155] structural formula
[0156] Molecular formula C 16 h 25 NO 3
[0157] Molecular weight 279.379
[0158] Appearance White to off-white crystalline powder
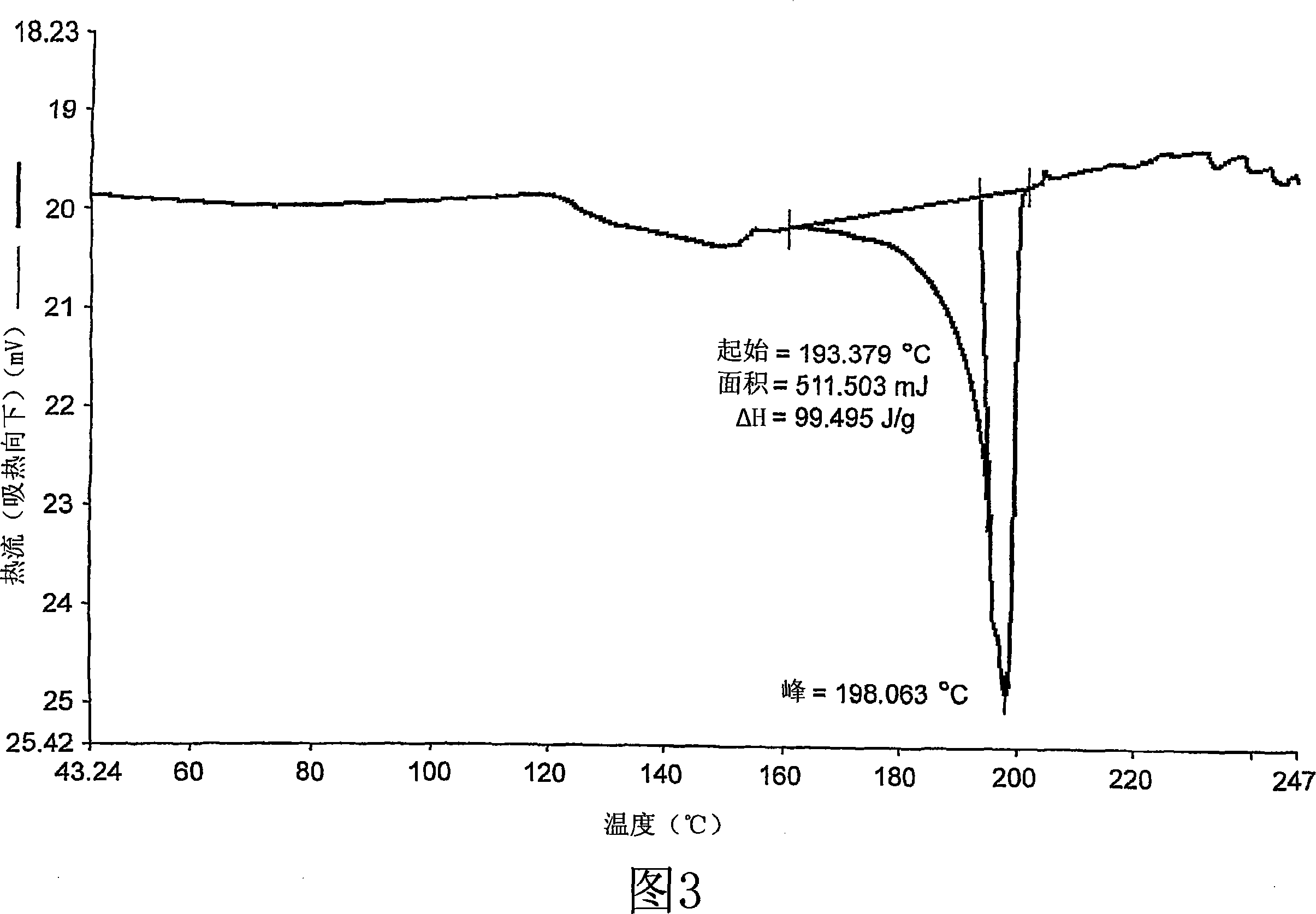
[0159] Melting point (DSC start) about 193.3790°C
[0160] X-ray (powder diffraction) one polymorph
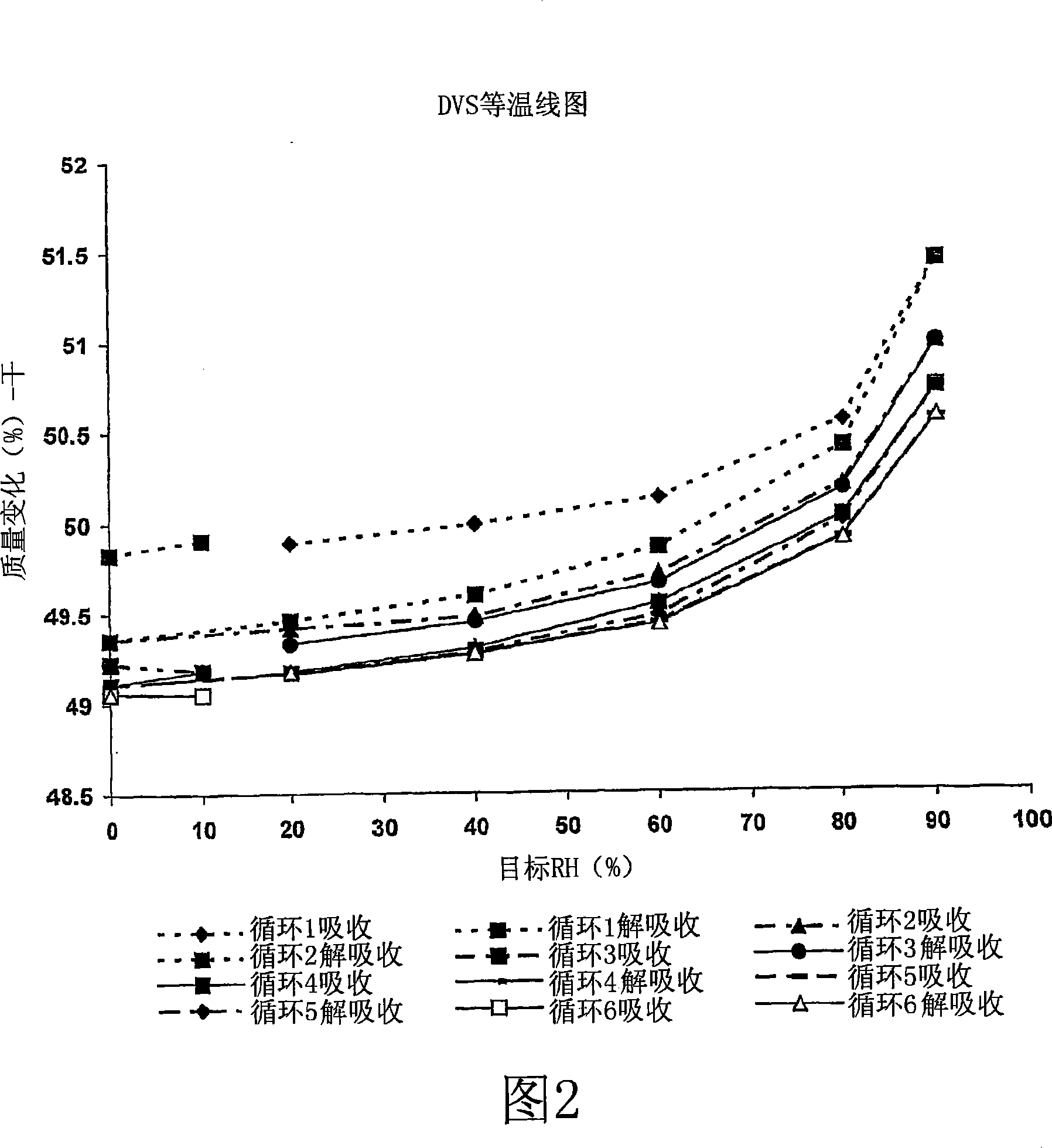
[0161] Hygroscopicity Non-hygroscopic (weight gain less than 2% at 26.30C / 90%RH, at %RH
[0162] No weight gain after lowering)
[0163] Solution Stability Compound in all aqueous solutions (pH 1.4-10.0) for at least 24 hours at room temperature
[0164] Stablize.
[0165] pH-Solubility Final pH 1.4 24.2mg / ml
[0166] Final...
example 3
[0169] Example 3: Salt Form of 1-[-2-Dimethylamino-1-(4-Phenol)ethyl]-cis-1,4-Cyclohexanediol
[0170] A. Succinate
[0171] 0.5008 g of 1-[-2-dimethylamino-1-(4-phenol)ethyl]-cis-1,4-cyclohexanediol was dissolved in 3 ml of acetone. The solution was heated to 60°C. 0.206 g of succinic acid (Sigma-Aldrich) was dissolved in 7 ml of acetone with 2 drops of water and heated to 70° C. in a water bath. The succinic acid solution was added dropwise to the 1-[-2-dimethylamino-1-(4-phenol)ethyl]-cis-1,4-cyclohexanediol solution with stirring at 70°C. Heating was continued at 65°C with the addition of a few drops of water to produce a single phase solution. Mixing was continued at 60°C for 10 minutes, then cooled to room temperature overnight. The precipitate formed at the bottom of the flask was dissolved in ethanol, and then the ethanol solution was evaporated under reduced pressure with a rotary evaporator to yield 0.4898 g of white powder.
[0172] 1 HNMR confirmed the struct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com