Preparation process of high-purity scutellarin bulk drug
A technology for breviscapine and preparation technology, which is applied in the field of preparation technology of high-purity raw materials, can solve problems such as incomplete removal, and achieve the effects of easy separation and convenient automatic control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
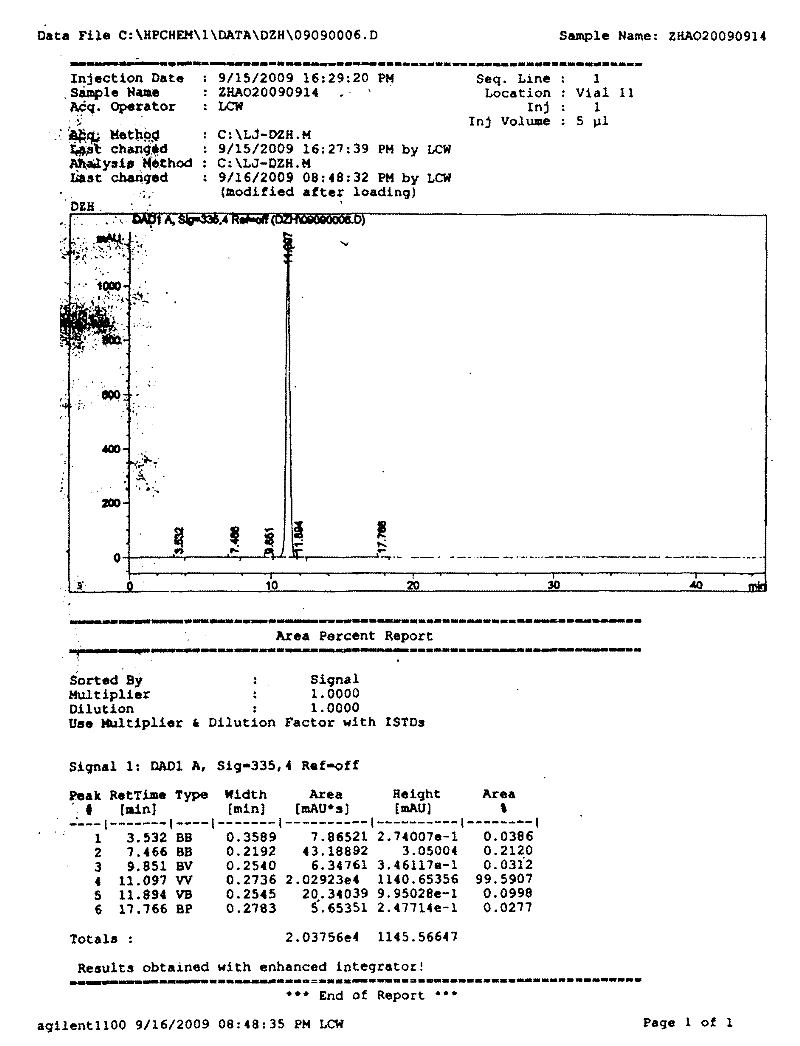
[0026]Example 1: Weigh 10kg of crude raw material with scutellarin content of 89.25%, add 50L of water and stir, add 38L of 10% arginine solution and heat to boiling, add water to make 100L aqueous solution, filter out insoluble matter, and filtrate while hot Add 50L of 10% sodium dihydrogen phosphate solution while stirring, then add 450L of acetone while stirring to make scutellarin crystallize, after standing for 3 hours, filter, drain the acetone, add 150L of water to boil and discard Remaining organic solvent, when the solution is cooled to below 60°C, add 25L of 20% glacial acetic acid solution, let it stand for 2 hours, filter it with suction, and dry it to obtain 6.25kg of dry product; it is the raw material of high-purity scutellarin. The purity of B as determined by HPLC is 99.5907%, such as figure 1 shown.
Embodiment 2
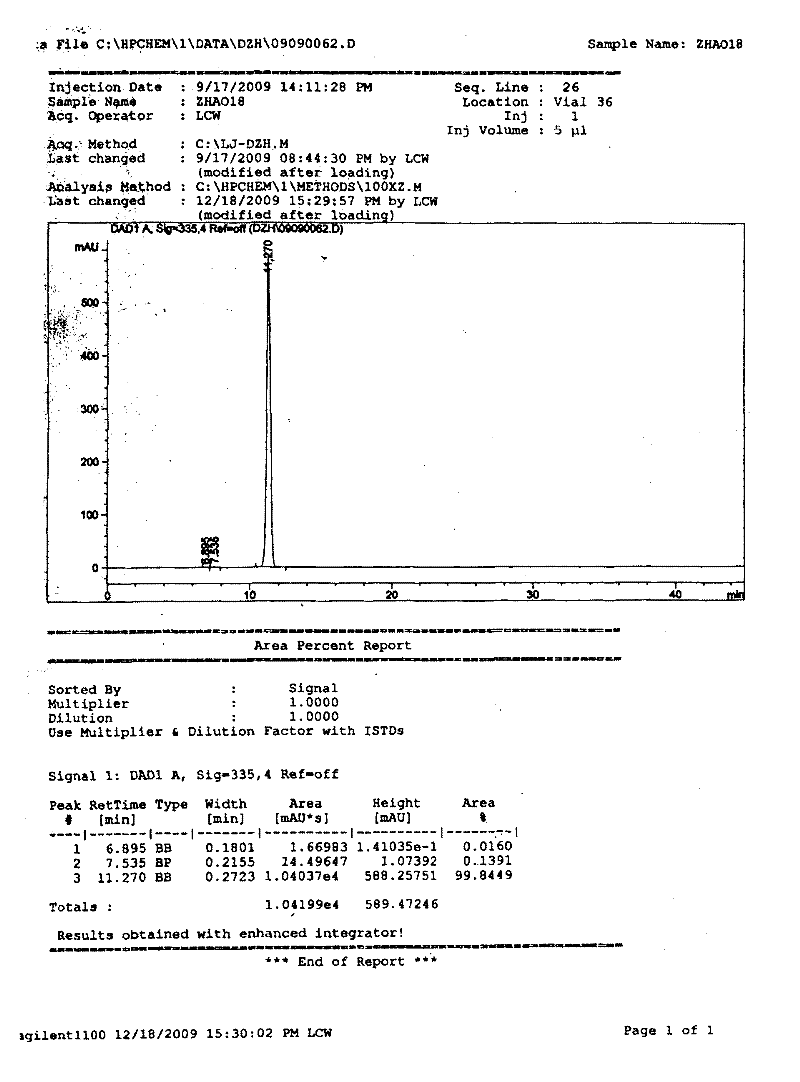
[0027] Example 2: Weigh 20kg of crude raw material with scutellarin content of 84.24%, add 100L of water and stir, add 38L of 20% arginine solution and heat to boiling, add water to make 200L aqueous solution, filter out insoluble matter, and put the model on the filtrate It is AB-8 macroporous resin column chromatography. After discarding the pigment and macromolecular substances, collect the eluate and concentrate it to about 200L by distillation. Add 100L of 10% potassium dihydrogen phosphate solution while stirring the concentrated solution while it is hot. Then add 800L of acetone while stirring to crystallize scutellarin. After standing for 3 hours, filter, drain the acetone, add 200L of water to boil and discard the residual organic solvent. When the solution is cooled to below 60°C, add 50L of 20% acetic anhydride solution was left to stand for 2 hours, suction filtered, and dried to obtain 11.61kg of dry product, which was the raw material of high-purity scutellarin. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com