Hepatitis B virus dendritic cell therapeutic vaccine and preparation method thereof
A technology of dendritic cells and therapeutic vaccines, applied in the field of therapeutic vaccines and their preparation, can solve the problems of insufficient specific cellular immune activation, peripheral immune tolerance, and low immune response, and achieve efficient induction of antiviral replication, The effect of promoting T cell immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] Hereinafter, preferred embodiments of the present invention will be described in detail with reference to the accompanying drawings. The experimental method that does not indicate specific conditions in the preferred embodiment is usually according to conventional conditions, such as described in the Molecular Cloning Experiment Guide (Third Edition, J. Sambrook et al., translated by Huang Peitang, etc., Science Press, 2002) conditions, or as recommended by the manufacturer.
[0032] 1. Preparation of Dendritic Cell Therapeutic Vaccine of Hepatitis B Virus
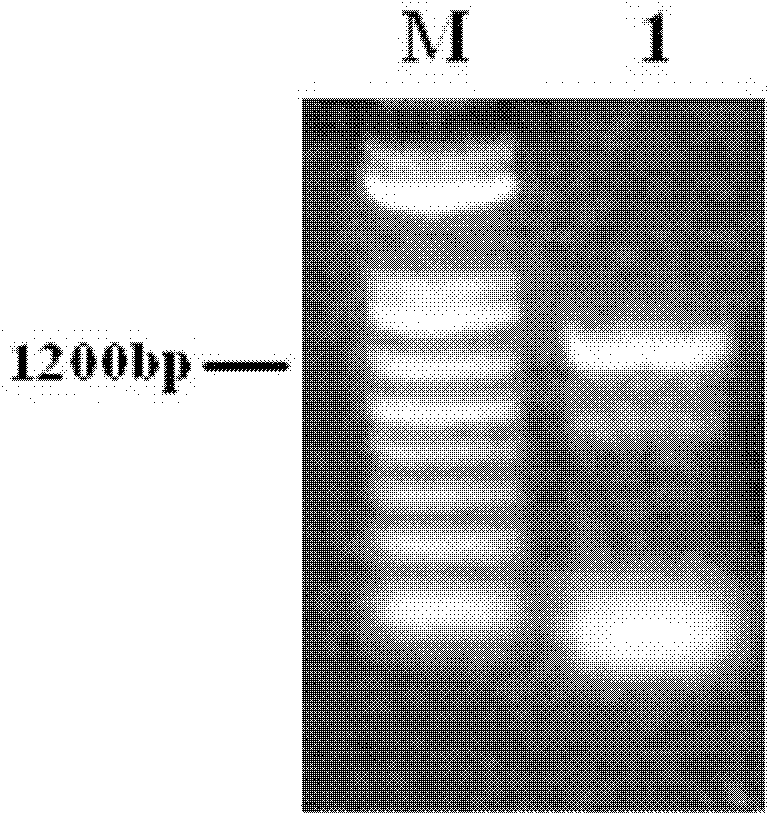
[0033] 1. Construction of recombinant eukaryotic vector for co-expression of HBcAg and Fc genes
[0034] (1) Cloning of HBcAg gene
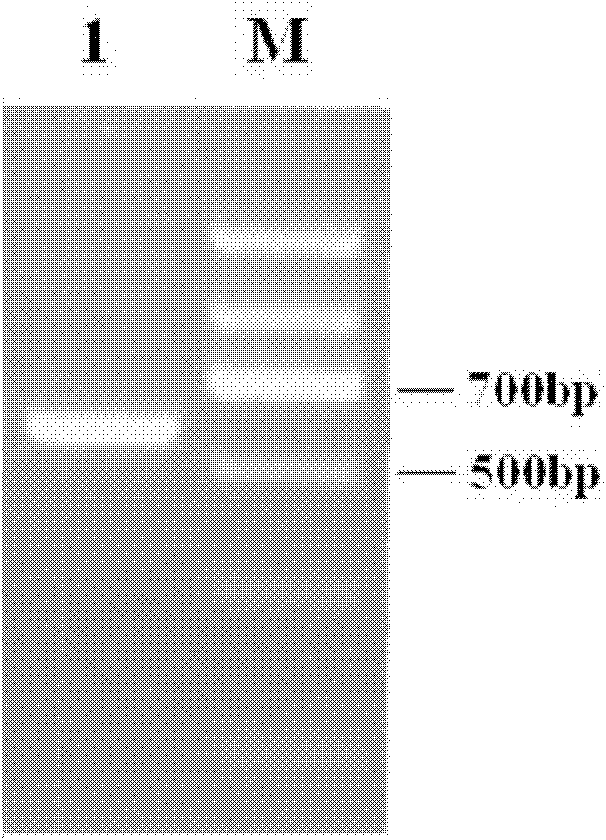
[0035] According to the HBcAg gene sequence with GenBank accession number NC_003977.1, the following primers were designed and synthesized to amplify the full-length HBcAg cDNA with restriction sites at both ends: F1: 5’-aat ctgcag ggcatggacatcgacccttat-3' (SEQ ID No: 3), the under...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com