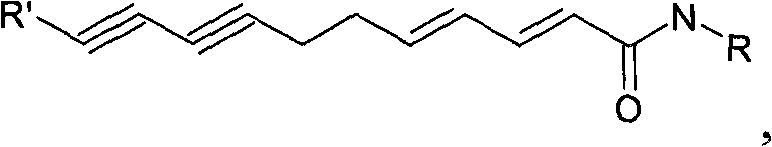
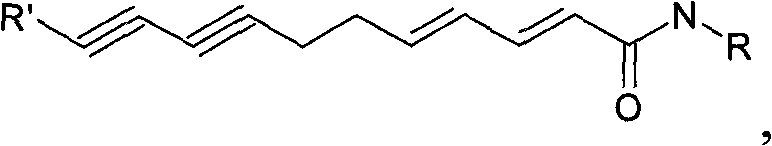
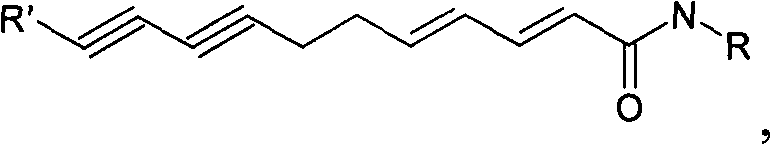
Flavor-enhancing amide compounds
A compound and composition technology, applied in the preparation of organic compounds, the preparation of carboxylic acid amides, organic chemistry, etc., can solve the problems of high volatility and strong
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0046]
[0047] Pent-4-yn-1-ol 5-iodopent-4-yn-1-ol
[0048] Preparation of 5-iodopent-4-yn-1-ol: Potassium hydroxide (115 g, 2.051 mol, purchased from Sigma-Aldrich) was dissolved in water (150 ml) and cooled to 0°C. Pent-4-yn-1-ol (69 g, 820 mmol, purchased from Sigma-Aldrich) was dissolved in methanol (1.125 L) and slowly added to the reaction mixture while maintaining the temperature at 0 °C. After 15-30 minutes, iodine (229 g, 902 mmol) was added in one portion, and the mixture was warmed to room temperature and stirred for 3 hours. The mixture was then diluted with water (750 mL), and washed with diethyl ether (Et 2 (2, 300 mL) was washed three times. The organic layers were combined and concentrated in vacuo to give a yellow oil. Dissolve the oil in dichloromethane (CH 2 Cl 2 ) (300mL), washed with brine (300mL), sodium sulfate (Na 2 SO 4 ) was dried and filtered. The solvent was removed in vacuo to give crude product (170 g), which was purified by column chr...
Embodiment II
[0051]
[0052] 5-Iodopent-4-yn-1-ol 7-(Trimethylsilyl)heptacarbon-4,6-diyn-1-ol
[0053] Preparation of 7-(trimethylsilyl)heptacarbon-4,6-diyn-1-ol: ethynyltrimethylsilane (112g, 1.143mol, purchased from Sigma-Aldrich Company), piperazine Pyridine (847ml, 8.571mol, purchased from Sigma-Aldrich) and 5-iodopent-4-yn-1-ol (120g, 0.571mol, prepared as described above) were combined and cooled to 0°C. Copper(I) chloride (5.66 g, 57.1 mmol, purchased from Sigma-Aldrich) was added in one portion. The reaction mixture was allowed to warm to room temperature. After 30 minutes, with saturated ammonium chloride solution (NH 4 Cl) (2.5L) to stop the reaction solution, and with Et 2 O (300 mL) was washed three times. The organic layers were combined, washed twice with brine (500 mL), washed with Na 2 SO 4 Dry, filter and concentrate in vacuo with a rotary evaporator. The resulting crude product was purified by silica gel chromatography (Hex:EtOAc 6:1) to give 7-(trimethylsilyl)h...
Embodiment III
[0056]
[0057] 7-(trimethylsilyl)heptacarbon-4,6-diyn-1-ol 7-(trimethylsilyl)heptacarbon-4,6-diynal
[0058] Preparation of 7-(trimethylsilyl)heptacarbon-4,6-diyne aldehyde: Dimethylsulfoxide (DMSO) (76ml, 1.076mol) was added dropwise to oxalyl chloride (47.1ml, 538mmol) at -78°C , purchased from Sigma-Aldrich) CH 2 Cl 2 (750ml) solution. The mixture was stirred at -78°C for 20 minutes. 7-(Trimethylsilyl)heptacarbon-4,6-diyn-1-ol (50 g, 269 mmol, prepared as above) was dissolved in CH 2 Cl 2 (15 mL) and added slowly. The mixture was stirred at -78°C for 1 hour. Triethylamine (225ml, 1.614mol, purchased from Sigma-Aldrich) was added. The reaction mixture was stirred for an additional 80 minutes while slowly warming to room temperature. with saturated NH 4 The reaction mixture was quenched with Cl, separated, and the aqueous portion was washed with CH 2 Cl 2 (200 mL) back-extracted twice. The combined organic layers were washed with Na 2 SO 4 Dry, filter and co...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap