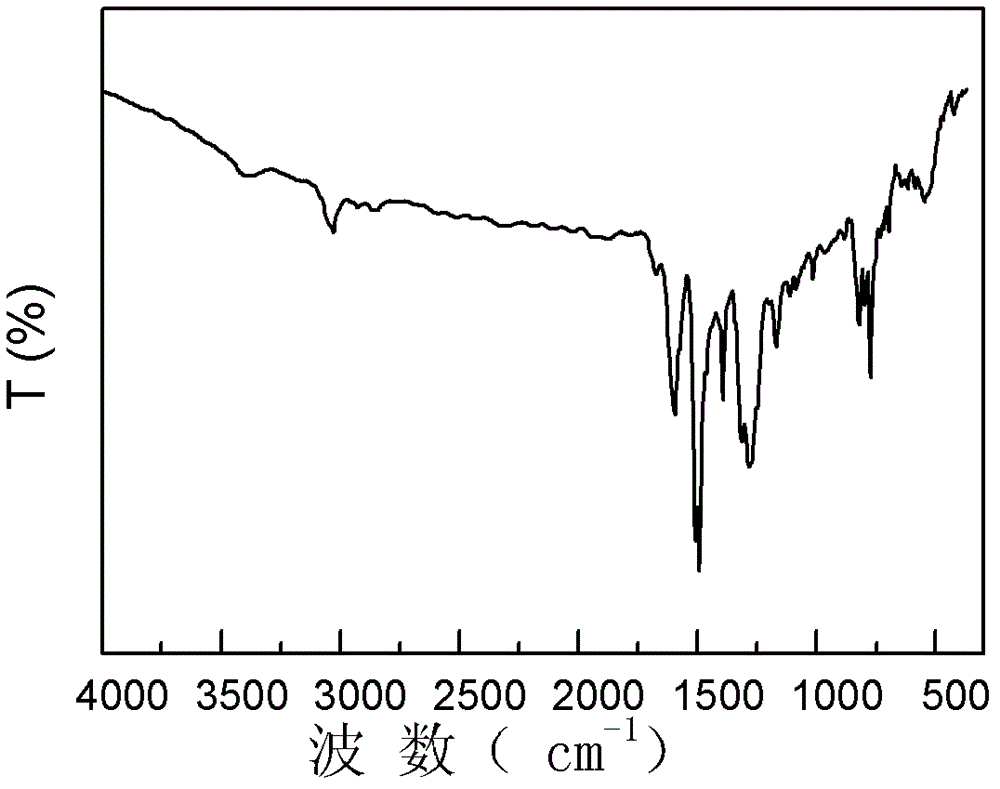
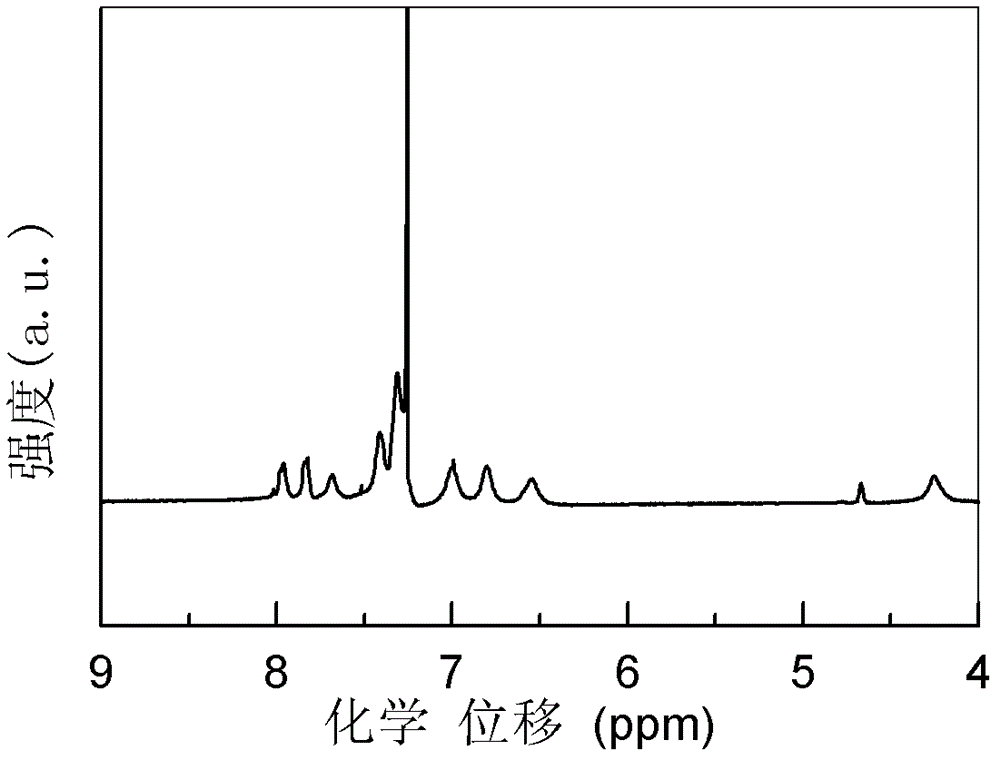
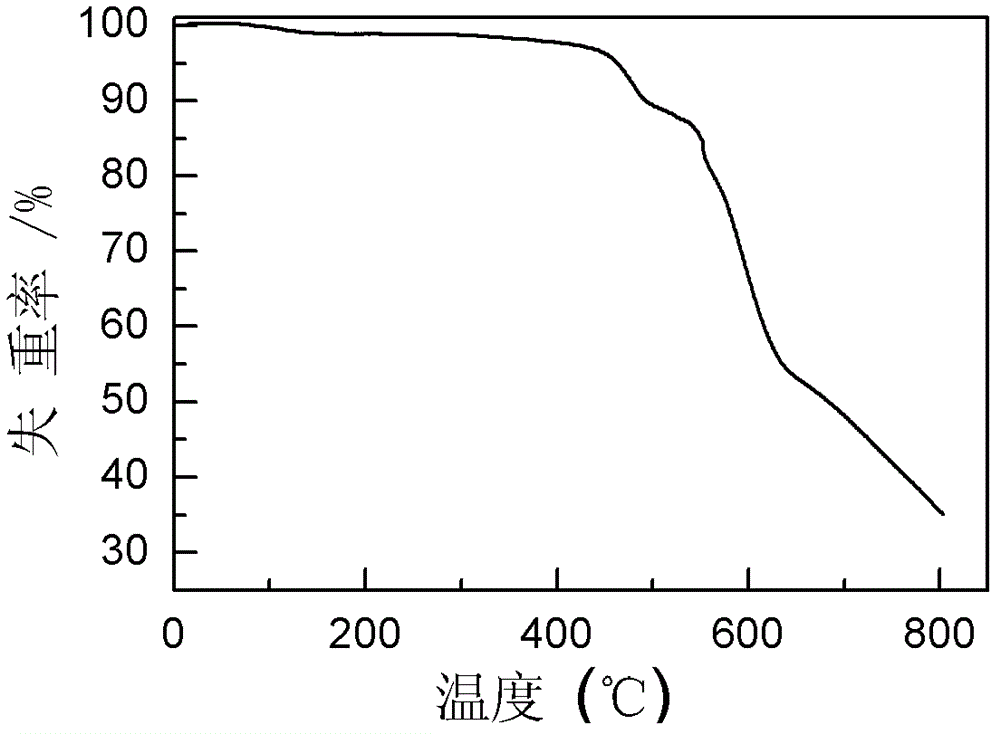
Polyaniline derivative, preparation method of reductive poly Schiff base of polyaniline derivative and application of polyaniline derivative
A polySchiff base, polyaniline technology, applied in chemical instruments and methods, optics, instruments, etc., can solve the problems of difficult to process into film, polyaniline structure and function is single, low solubility, etc., to achieve improved solubility, Increased solubility, high sensitivity to color change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0045] Specific embodiment one: the structural formula of the polyaniline derivative of the present embodiment is as follows:
[0046] In the formula, n is a positive integer, and R is where R″ is (CH 2 ) p CH 3 , p is 0-8, R' is H or CH 3 , m is 1-8.
[0047] The polyaniline derivatives of this embodiment use propeller-type triarylamine as a monomer, which can effectively reduce the strong force between polymer molecular chains and increase the solubility of the polymer. At the same time, triarylamine is easy to form cationic radicals and presents a Different from the triarylamine in the neutral state, and because the structure contains two monomer units, the polyaniline derivatives have color tunability due to the joint action; on the other hand, the synthesis of the usual light-emitting or color-changing polymers requires expensive raw materials , harsh synthesis conditions, and the present invention further increases its solubility by reducing the poly-Schiff b...
specific Embodiment approach 2
[0048] Embodiment 2: This embodiment is different from Embodiment 1 in that n is an integer ranging from 3 to 10. Others are the same as in the first embodiment.
[0049] Embodiment 3: The difference between this embodiment and specific embodiment 1 or 2 is that or R" in (CH 2 ) p CH 3 , p is 2-6. Others are the same as in the first or second embodiment.
specific Embodiment approach 4
[0050] Specific implementation mode four: the difference between this implementation mode and one of the specific implementation modes one to three is that m in is 3-5. Others are the same as those in the first to third specific embodiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com