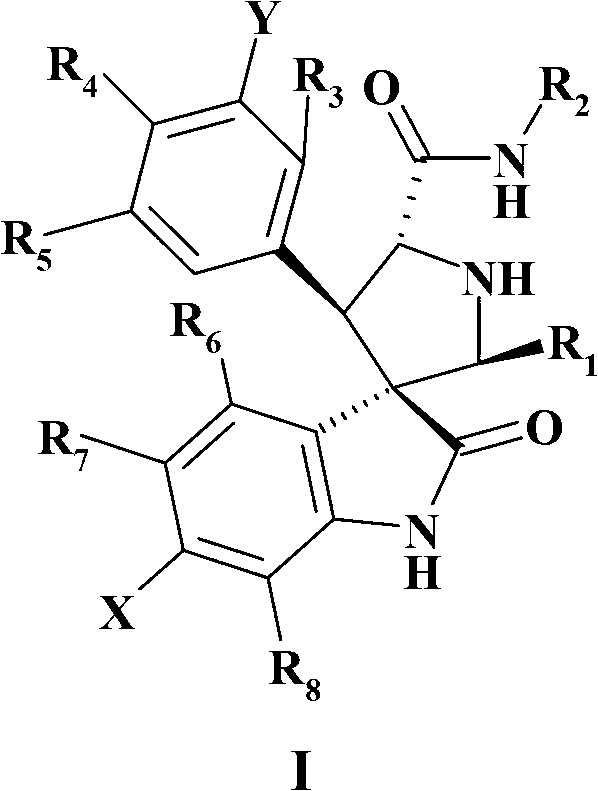
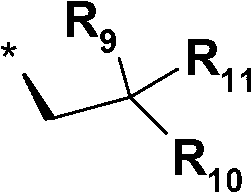
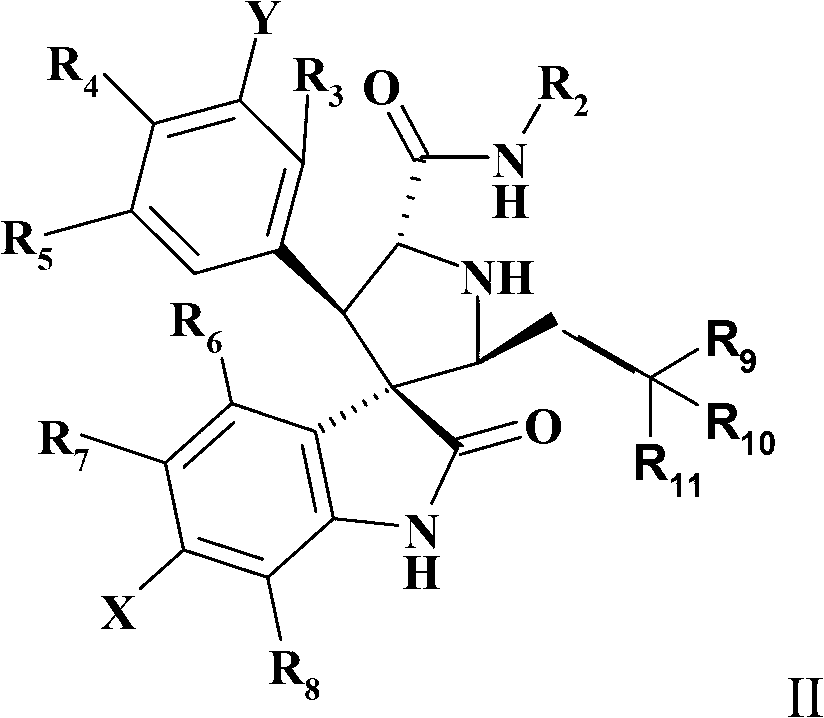
Spiroindolinone pyrrolidines
A technology of pyrrolidine and alkyl, applied in organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve problems affecting MDM2 protein degradation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0287] Preparation of intermediate [3,3-dimethyl-but-(E)-imino]-tert-butyl acetate
[0288]
[0289] M.W.213.32 C 12 H 23 NO 2
[0290] Combine tert-butyl glycine (Alfa) (2.71g, 20.0mmol) and 3,3-dimethyl-butyraldehyde (Alfa) (2.21g, 21.0mmol) in CH 2 Cl 2 (50 mL) was stirred at room temperature overnight. The reaction mixture was concentrated and the residue was dried in vacuo to give [3,3-dimethyl-butyl-(E)-iminoamino]-acetic acid tert-butyl ester (4.29 g, 100%) as a colorless oil The oil was used in the next step without further purification.
Embodiment 2
[0292] Preparation of intermediate E / Z-6-chloro-3-(3-chloro-2-fluoro-benzylidene)-1,3-dihydro-indol-2-one
[0293]
[0294] M.W.308.14 C 15 H 8 Cl 2 FNO
[0295] To a mixture of 6-chloro-2-hydroxyindole (11g, 65.6mmol) (Crescent) and 3-chloro-2-fluorobenzaldehyde (12g, 75.7mmol) (Aldrich) in methanol (140mL) was added dropwise Pyridine (5.59 g, 65.6 mmol) (Aldrich). The mixture was then heated at 50°C for 3 hours. After cooling to 4°C, the mixture was filtered and the resulting precipitate was collected and dried to obtain E / Z-6-chloro-3-(3-chloro-2-fluoro-benzylidene)-1,3-dihydro- Indole-2-one, which is a yellow solid (yield 18 g, 89%).
Embodiment 3
[0297] Intermediate racemic-(2'S,3'R,4'S,5'R)-6-chloro-4'-(3-chloro-2-fluoro-phenyl)-2'-(2,2-dimethyl -Propyl)-2-oxo-1,2-dihydro-spiro[indole-3,3'-pyrrolidine]-5'-carboxylic acid tert-butyl ester
[0298]
[0299] M.W.521 C 27 H 31 Cl 2 FN 2 O 3
[0300] To [3,3-dimethyl-butyl-(E)-imino]-tert-butyl acetate (3.37g, 15.8mmol) prepared in Example 1 and E / Z-6 prepared in Example 2 -Chloro-3-(3-chloro-2-fluoro-benzylidene)-1,3-dihydro-indol-2-one (4g, 13mmol) in dichloromethane (100mL) was added three Ethylamine (6.6mL, 47.4mmol) and AgF (2g, 15.8mmol). The mixture was stirred at room temperature for 18 hours. The mixture was concentrated and the residue was partitioned between ethyl acetate and brine. Separate the organic layer, wash with water and use MgSO 4 Dry and concentrate. The residue was dissolved in tert-butanol (30 mL) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, Fluka) (7.2 g, 47.4 mmol) was added. The reaction mixture was heated at 120°C for 2 hours. The mixture was ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap