Therapeutic agent (Y - 39983) for corneal endothelial dysfunction
A technology for corneal endothelial dysfunction, applied in the direction of cell culture active agents, medical preparations containing active ingredients, nervous system cells, etc., can solve the in vivo effects of Y-27632 and fasudil, and the impact of corneal endothelial cells Issues not considered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0197] Preparation of corneal endothelial cell layer
[0198] The cell suspension of corneal endothelial cells can be prepared according to collection and in vitro culture of corneal endothelial cells and subculture of the above-mentioned corneal endothelial preparation. The cell suspension is seeded onto a substrate such as a collagen sheet or the like, and cultured. Here, the number of seeded cells is controlled so that the corneal endothelial preparation finally produced has a cell layer with a desired cell density. Specifically, cells are seeded to form cells with about 1,000 to about 4,000 cells / mm 2 The cell layer of the cell density. The culture can be performed under conditions similar to the above-mentioned primary culture and the like. Although the culture time varies depending on the condition of the cells used, it is, for example, 3 to 30 days. By adding the compound of the present invention, preferably compound (Ia) ((R)-(+)-N-(1H-pyrrolo[2,3-b]pyridin-4-yl)...
Embodiment 1
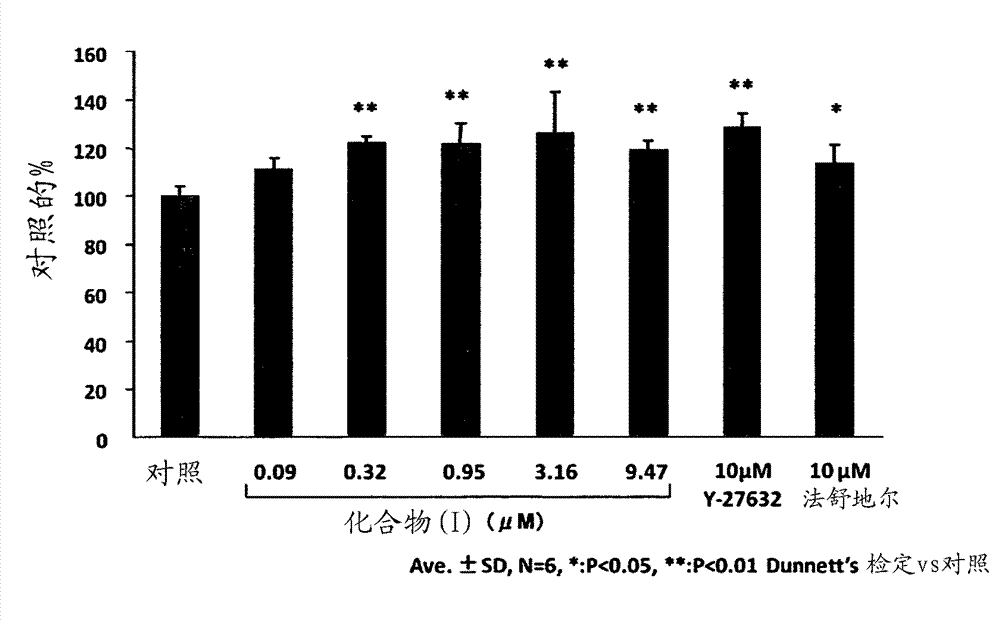
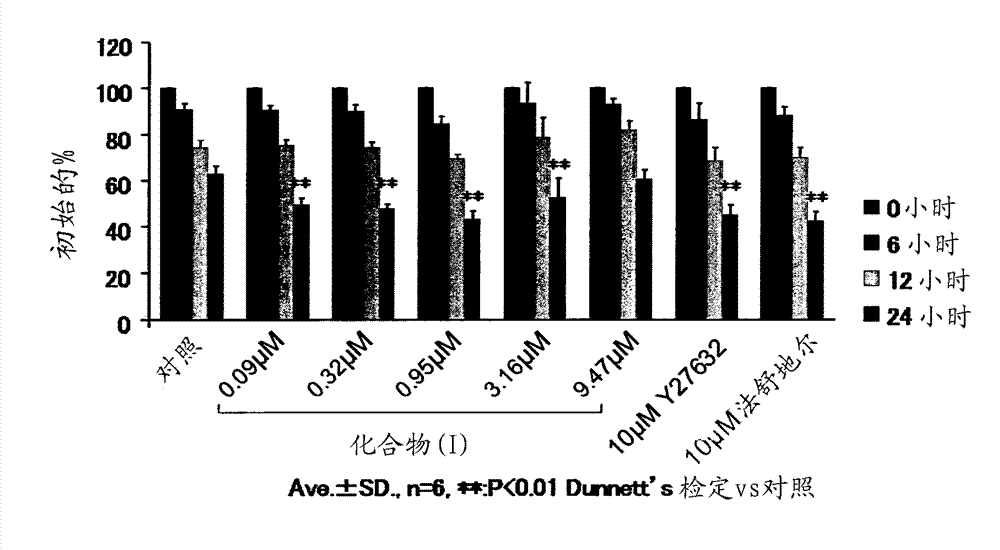
[0228] Embodiment 1: the effect of compound (I) in rabbit corneal endothelial wound model
[0229] 1. Test substances and reference substances
[0230] As a test substance, (R)-(+)-N-(1H-pyrrolo[2,3-b]pyridin-4-yl)-4-(1-aminoethyl)benzamide monohydrochloride was used (compound (I)). Compound (I) was prepared according to the methods described in WO95 / 28387 and WO2002 / 083175.
[0231] In this example, a 0.95 mM (0.03 w / v %) compound (I) infusion obtained by diluting the above-mentioned infusion with the above-mentioned excipient and 0.32 mM (0.01 w / v %) of the compound (I) were used Instillation.
[0232] As positive control substances, Y-27632 dihydrochloride (Wako Pure Chemical Industries, Co., Ltd., Cat. #253-00513) and hydrated fasudil hydrochloride injection (Eril (registered trademark) intravenous Injection solution, Asahi Kasei Pharma) was instilled, and Y-27632 and fasudil were each adjusted to 10 mM with phosphate buffered saline (PBS, Invitrogen, Cat. #14190). In...
Embodiment 2
[0254] Embodiment 2: the effect of administration of high concentration compound (I) on rabbit corneal endothelial wound model
[0255] The effect could be demonstrated in a rabbit corneal endothelial loss model by instillation of 0.05 w / v% (1.58 mM) of compound (I) (topical instillation; 6 times a day for 2 days).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com