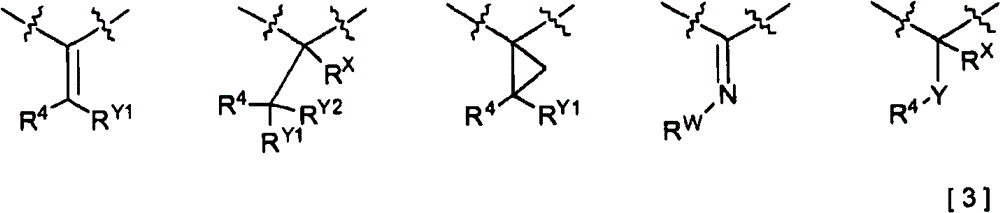
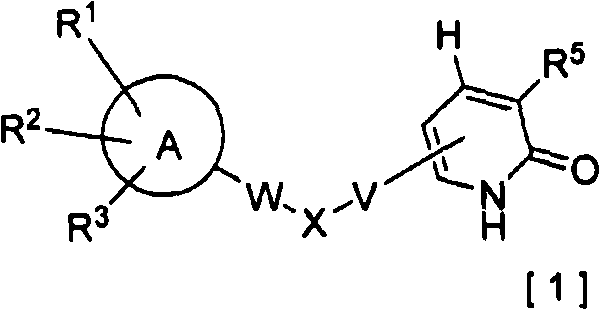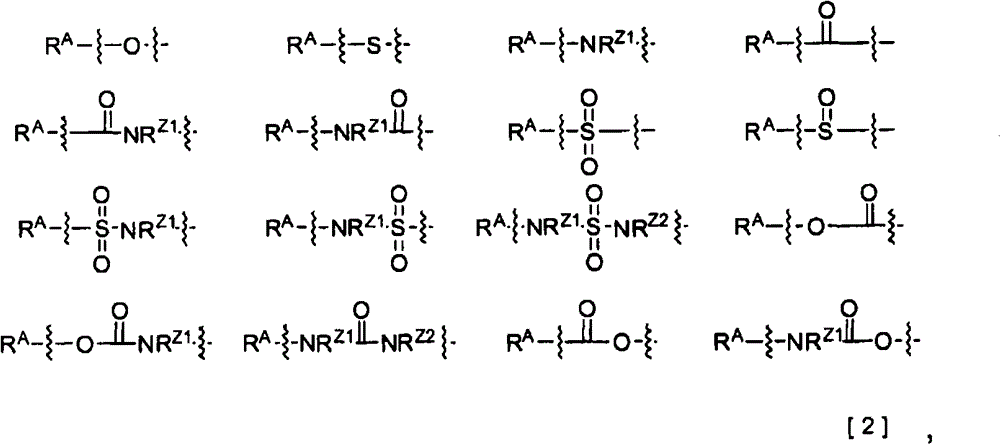2-pyridone compound
A pyridone and compound technology, applied in the field of novel 2-pyridone compounds, can solve problems such as reducing GK activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1-2
[0818] (5-Chloro-6-methoxypyridin-2-yl)[4-(cyclopropylsulfanyl)phenyl]methanone
[0819] 1 HNMR (300MHz, CDCl 3 ) δppm 0.61-0.83 (m, 2H), 1.07-1.22 (m, 2H), 2.23 (tt, J=7.3, 4.4Hz, 1H), 4.02 (s, 3H), 7.43 (d, J=8.7Hz , 2H), 7.63(d, J=7.8Hz, 1H), 7.82(d, J=7.9Hz, 1H), 8.09(d, J=8.7Hz, 2H).
[0820] MS(+): 342 [M+Na] + .
reference example 1-3
[0822] (5-Chloro-6-methoxypyridin-2-yl)[4-(cyclopentylsulfanyl)phenyl]methanone
[0823] 1 HNMR (300MHz, CDCl 3 )δppm1.58-1.74 (m, 4H), 1.75-1.91 (m, 2H), 2.08-2.30 (m, 2H), 3.61-3.85 (m, 1H), 4.02 (s, 3H), 7.34 (d, J=8.7Hz, 2H), 7.63(d, J=7.8Hz, 1H), 7.81(d, J=7.8Hz, 1H), 8.07(d, J=8.7Hz, 2H).
[0824] MS(+): 348[M+H] + .
[0825] Reference example 1-4
[0826] [3-Chloro-4-(ethylsulfanyl)phenyl](5-chloro-6-methoxypyridin-2-yl)methanone
[0827] 1 HNMR (300MHz, CDCl 3 )δppm1.45(t, J=7.4Hz, 3H), 3.06(q, J=7.3Hz, 2H), 4.04(s, 3H), 7.27(d, J=8.4Hz, 1H), 7.68(d, J=7.8Hz, 1H), 7.83(d, J=7.9Hz, 1H), 8.04(dd, J=8.4, 1.9Hz, 1H), 8.31(d, J=1.9Hz, 1H).
[0828] Reference example 1-5
[0829] (5-Chloro-6-methoxypyridin-2-yl)[3-(cyclopropylsulfanyl)phenyl]methanone
[0830] 1 HNMR (300MHz, CDCl 3 ( m, 1H), 7.65(d, J=7.8Hz, 1H), 7.82(d, J=7.8Hz, 1H), 7.86(dt, J=7.7, 1.3Hz, 1H), 8.10(t, J=1.6 Hz, 1H).
[0831] MS(+): 320[M+H] + .
[0832] Reference example 1-6
[0833] (5...
reference example 1-10
[0849] (5-Chloro-6-methoxypyridin-2-yl){4-[(3-methylbutoxy)methyl]phenyl}methanone
[0850] 1 HNMR (300MHz, CDCl 3 )δppm0.92(d, J=6.6Hz, 6H), 1.50-1.60(m, 2H), 1.67-1.84(m, 1H), 3.54(t, J=6.7Hz, 2H), 4.00(s, 3H ), 4.58(s, 2H), 7.44(d, J=8.3Hz, 2H), 7.64(d, J=7.7Hz, 1H), 7.81(d, J=7.7Hz, 1H), 8.10(d, J =8.0Hz, 2H).
[0851] MS(+): 348[M+H] + .
[0852] Reference example 1-11
[0853] (5-Chloro-6-methoxypyridin-2-yl)[4-(propan-2-yl)phenyl]methanone
[0854] 1 HNMR (300MHz, CDCl 3 )δppm1.29(s, 3H), 1.31(s, 3H), 2.92-3.06(m, 1H), 4.03(s, 3H), 7.33(d, J=8.4Hz, 2H), 7.61(d, J =7.8Hz, 1H), 7.80(d, J=7.8Hz, 1H), 8.09(d, J=8.7Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com