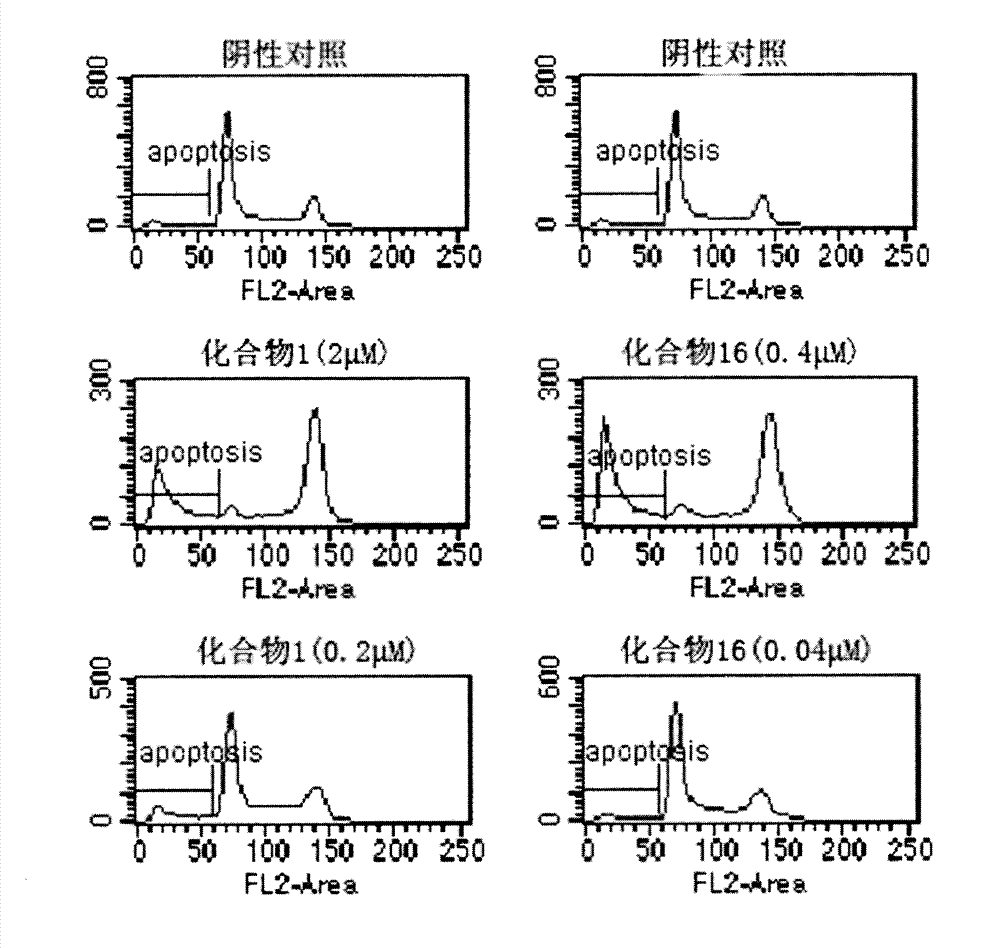
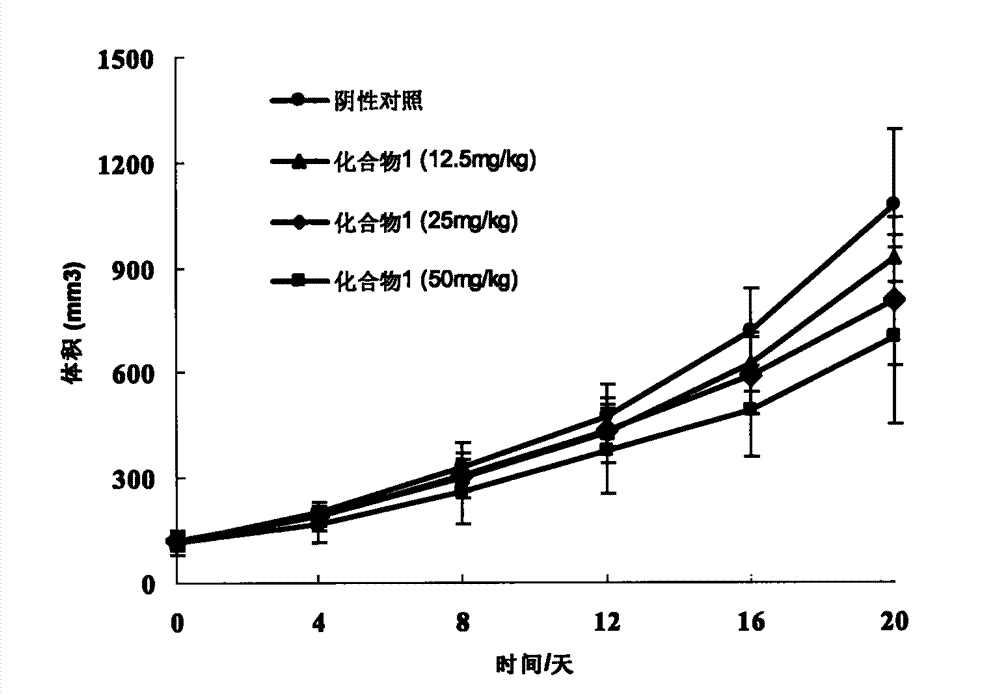
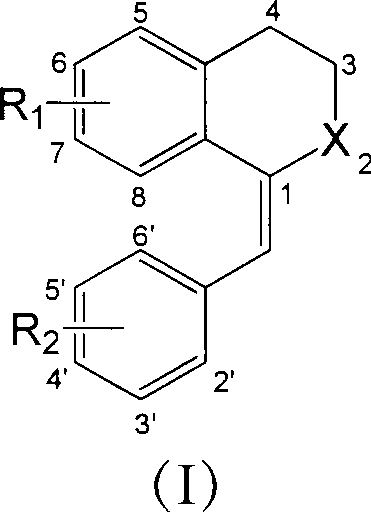
1-substituted benzylidene-2-naphthalenone derivative, preparation method thereof and use thereof
A technology of benzylidene and substituents, which is applied in the preparation of carbon-based compounds, organic compounds, active ingredients of ketones, etc., can solve problems such as drug resistance and damage to normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: the preparation of 6-methoxy-3,4-dihydro-2-naphthone
[0066] 2,6-Dimethoxynaphthalene (20g, 0.1mol) was added to refluxing dehydrated ethanol (200ml), added shredded metal sodium wire (19g, 0.83mol), and refluxed until the metal sodium was completely dissolved for about 1 Hours, down to room temperature, hydrochloric acid (2M, 330ml) was added dropwise in a water bath to adjust the pH=3, and the temperature was continued to rise and reflux for half an hour until clarification. Cool down to room temperature, extract three times with ethyl acetate (50ml), concentrate the aqueous phase, then extract three times with ethyl acetate (50ml), combine the organic phases, wash with saturated brine, and dry over anhydrous sodium sulfate. After concentration and high vacuum distillation, a yellow oil (boiling point 160-165°C, 10mmHg) was obtained, which was recrystallized from petroleum ether (60-90°C) to obtain a white needle-like solid with a yield of 75% and a mel...
Embodiment 2
[0067] Example 2: Preparation of (E)-6-methoxy-1-(3,4,5-trimethoxybenzylidene)-3,4-dihydro-2-naphthalenone (1)
[0068] Dissolve 6-methoxy-3,4-dihydro-2-naphthalenone (1.76g, 10mmol), 3,4,5-trimethoxybenzaldehyde (1.96g, 10mmol) in dichloromethane (30ml) Add 4A molecular sieves (3g), glacial acetic acid (50mg), piperidine (50mg), stir at room temperature for 8 hours, filter, wash the filter cake, combine the organic phases, wash with water (25ml), wash with saturated brine (25ml), anhydrous It was dried over sodium sulfate, concentrated, separated by column chromatography (PE:EtOAc=10:1), and recrystallized to obtain yellow crystals (1.68 g, 47%).
[0069] m.p.102-103°C.
[0070] 1 H NMR (300MHz, CDCl 3 )δ7.49(s, 1H), 7.44(d, J=8.6Hz, 1H), 6.80(d, J=2.2Hz, 1H), 6.72(s, 2H), 6.64(dd, J=8.6, 2.2 Hz, 1H), 3.85(d, J=13.9Hz, 6H), 3.71(s, 6H), 3.01(t, J=6.4Hz, 2H), 2.62(t, J=6.4Hz, 2H).
[0071] 13 C NMR (300MHz, CDCl 3 )δ201.78, 159.42, 152.91, 140.23, 138.68, 133.21, 132.59...
Embodiment 3
[0073] Example 3: (E)-6-methoxy-1-(3,4,5-trimethoxybenzylidene)-1,2,3,4-tetrahydro-2-naphthyl alcohol (16) preparation of
[0074] Dissolve (E)-6-methoxy-1-(3,4,5-trimethoxybenzylidene)-3,4-dihydro-2-naphthalenone (2g, 5.6mmol) in 10 % dichloromethane methanol solution, sodium borohydride was added, stirred at room temperature for 20 minutes, concentrated, washed with water, extracted with ethyl acetate, washed with saturated brine, combined organic phases, concentrated, and separated by column chromatography (PE:EtOAc=5:1 ), recrystallized to give white needles (1.7g, yield 85%), m.p.128°C
[0075] 1H NMR (300MHz, CDCl 3 )δ7.60(d, J=9Hz, 1H), 7.00(s, 1H), 6.77-6.81(m, 3H), 6.68(d, J=2.7Hz, 1H), 5.05(t, J=2.44Hz , 1H), 3.93(m, 10H), 3.82(s, 3H), 3.10-3.22(m, 1H), 2.68-2.76(m, 1H), 2.01-2.15(m, 1H), 1.95-2.09(m , 1H)
[0076] 13 C NMR (300MHz, CDCl 3 )δ 159.11, 152.97, 139.31, 139.02, 136.90, 133.31, 131.39, 129.97, 124.60, 122.59, 112.72, 111.51, 106.54, 106.15, 72.58,...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap