1,2,4-triazole derivative, preparation method and application thereof and organic electroluminescent device
一种三氮唑类、衍生物的技术,应用在有机电致发光领域,能够解决发光效率不高、发展滞后等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
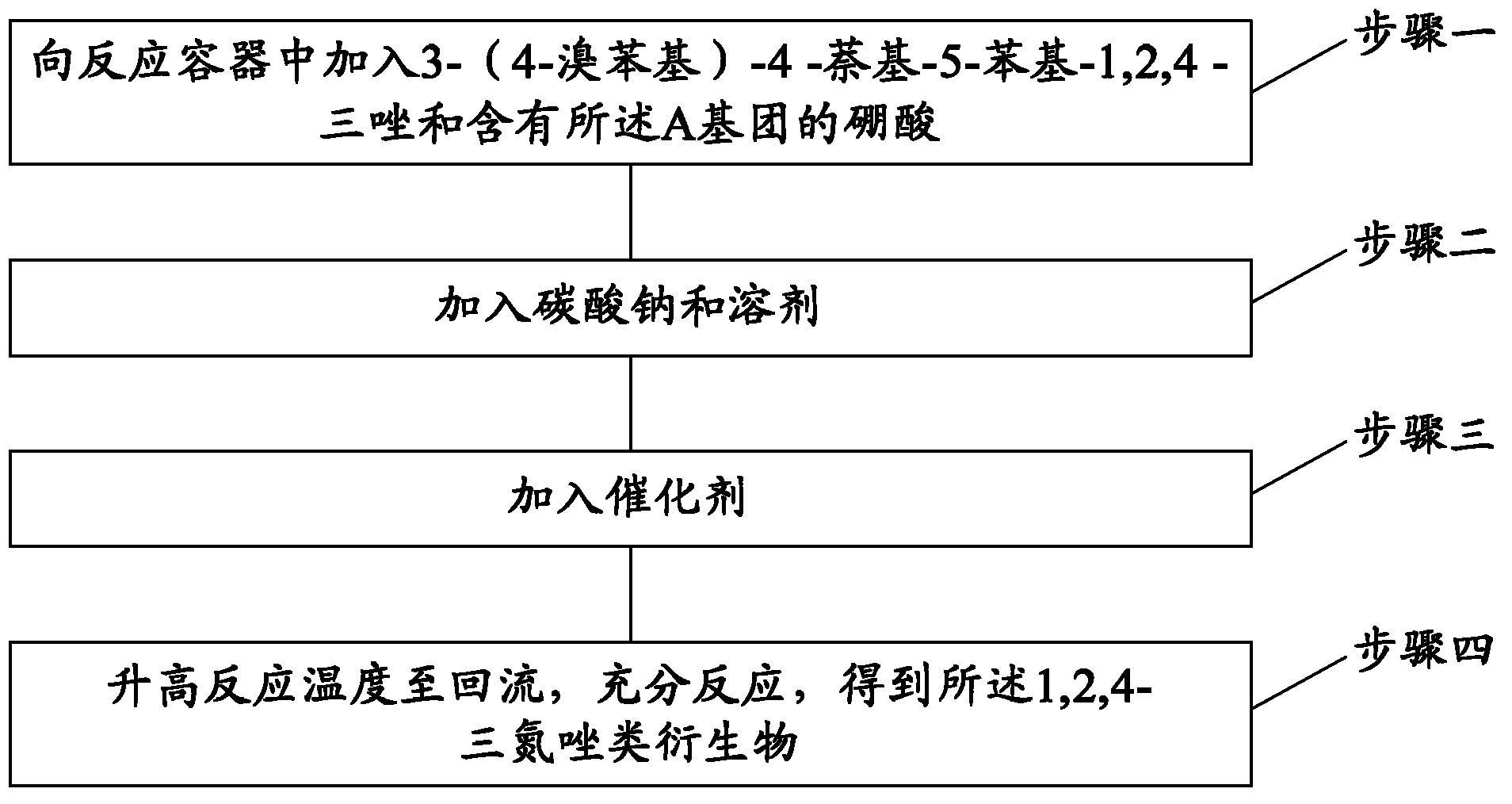
[0045] Corresponding to the above-mentioned 1,2,4-triazole derivatives, the embodiment of the present invention also provides a preparation method of 1,2,4-triazole derivatives, the reaction principle of this method is Suzuki (Suzuki) coupling reaction. Specifically, this method includes the following steps:
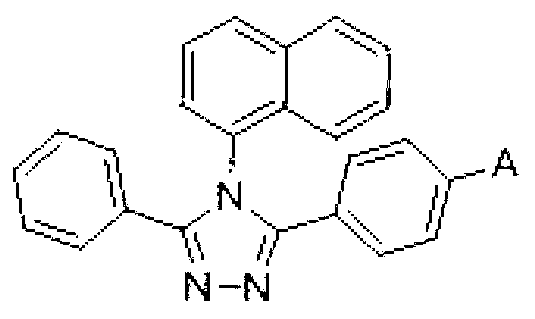
[0046] Step 1: Add 3-(4-bromophenyl)-4-naphthyl-5-phenyl-1,2,4-triazole and boronic acid containing the A group into the reaction vessel. In this step, weigh an appropriate amount of reactant 3-(4-bromophenyl)-4-naphthyl-5-phenyl-1,2,4-triazole and boronic acid containing the A group. Preferably, the mole fraction of 3-(4-bromophenyl)-4-naphthyl-5-phenyl-1,2,4-triazole and the boric acid containing the A group selected in this step They are: 3-(4-bromophenyl)-4-naphthyl-5-phenyl-1,2,4-triazole: 1 part; boric acid containing the A group: 1.5-2.5 parts. Further preferably, the molar fraction of 3-(4-bromophenyl)-4-naphthyl-5-phenyl-1,2,4-triazole and boronic acid contai...
Embodiment 1
[0069] Synthesis of compound 001:
[0070] Under nitrogen atmosphere, first add 21.32g (50mmol) of 3-(4-bromophenyl)-4-naphthyl-5-phenyl-1,2,4-triazole, N-phenyl-3-carbazole Add 21.53g (75mmol) of boronic acid into a three-necked flask equipped with a heating device, a reflux device and a stirring device, then add 15.90g (150mmol) of sodium carbonate, 250ml of toluene and 125ml of water, and finally add tetrakis(triphenylphosphine) Palladium 0.57g (0.5mmol) was added, the temperature was raised to 70°C, refluxed for 24 hours, cooled to room temperature, after the solid was precipitated, suction filtered, and the obtained filter cake was washed with water, ethanol and ether in sequence, and then subjected to column chromatography (Petroleum ether: ethyl acetate = 1:8) was purified, the mixed solvent was recovered, and dried to obtain 27.37g of off-white compound 001, with a yield of over 93%.
[0071] The specific synthetic route of compound 001 is as follows:
[0072]
[...
Embodiment 2
[0076] Synthesis of compound 002:
[0077] Under a nitrogen atmosphere, 21.32 g (50 mmol) of 3-(4-bromophenyl)-4-naphthyl-5-phenyl-1,2,4-triazole, 4-(4-triphenylamine)naphthalene Add 31.52g (90mmol) of boronic acid into a three-necked flask equipped with a heating device, a reflux device and a stirring device, then add 16.56g (160mmol) of sodium carbonate, 250ml of toluene and 125ml of water, and finally add tetrakis (triphenylphosphine) Palladium 0.69g (0.6mmol) was added, the temperature was raised to 75°C, refluxed for 25 hours, cooled to room temperature, after the solid was precipitated, suction filtered, and the obtained filter cake was washed with water, ethanol and ether in sequence, and then subjected to column chromatography (Petroleum ether: ethyl acetate = 1:8) was purified, the mixed solvent was recovered, and dried to obtain 27.47g of off-white compound 002, with a yield of over 93%.
[0078] The specific synthetic route of compound 002 is as follows:
[0079] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence quantum yield | aaaaa | aaaaa |
| fluorescence quantum yield | aaaaa | aaaaa |
| fluorescence quantum yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com