Manidipine hydrochloride compound
A technique for manidipine hydrochloride and compounds, applied in the field of manidipine hydrochloride compounds and their preparation, capable of solving the problems of unsatisfactory pharmacodynamics or pharmacokinetic performance of second-generation calcium antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
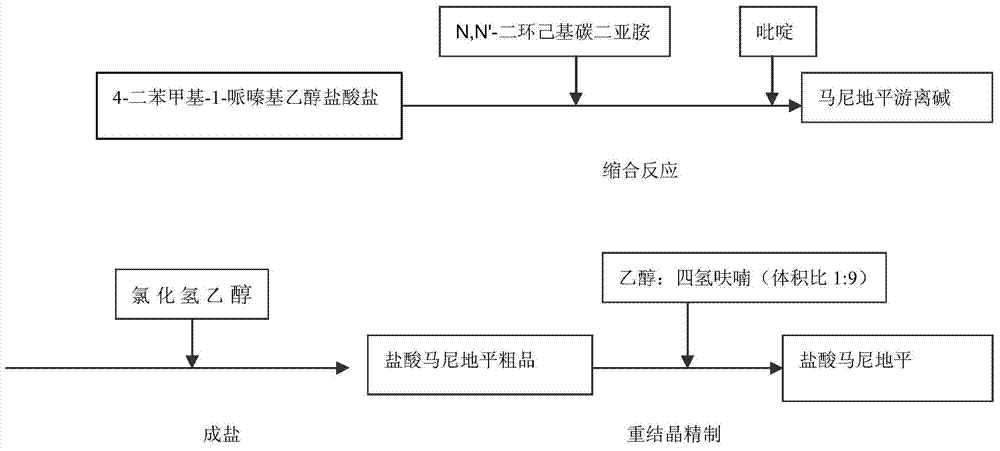
[0022] The synthetic route of manidipine compound of the present invention sees attached figure 1 .
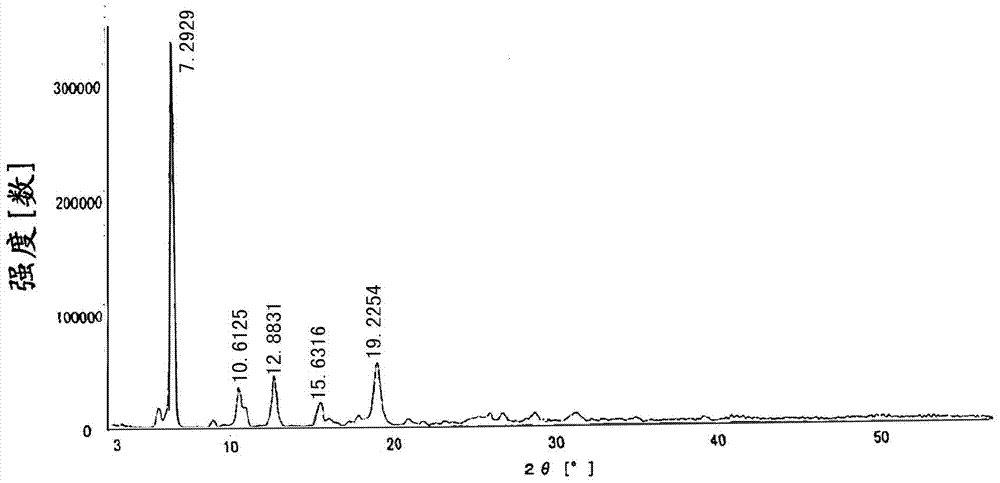
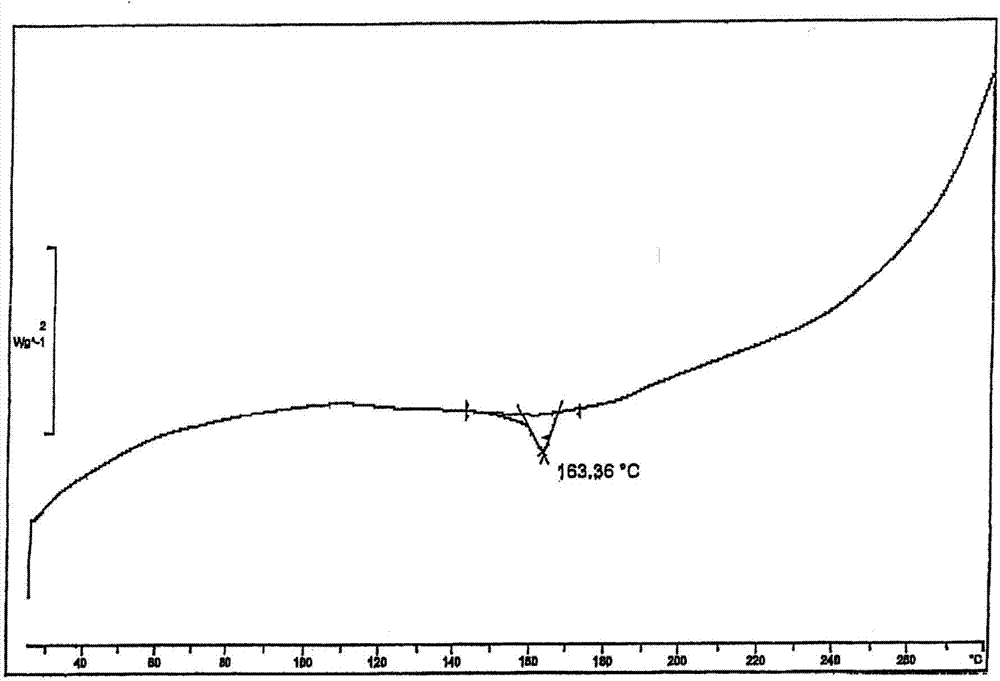
[0023] The total yield of the reaction is 81%. Its powder X-ray diffraction: using Cu-K radiation, the X-ray powder diffraction pattern is shown in the attached figure 2 ; Differential thermal analysis (DSC) diagram see attached image 3 .
Embodiment 2
[0024] The preparation of embodiment 2-compound tablet of the present invention
[0025] prescription:
[0026] Manidipine hydrochloride (embodiment 1)
10g
Hypromellose
2.5g
purified water
10g
lactose
80g
Poloxamer 188
7.5g
Magnesium stearate
0.5% of the total weight of the pellets
production
1000 pieces
[0027] Preparation method:
[0028] ① Pass manidipine hydrochloride through a 200-mesh sieve for later use, and pass lactose and hydroxypropyl methylcellulose through a 100-mesh sieve for later use;
[0029] ②Mix the prescription amount of lactose and hydroxypropyl methylcellulose evenly in equal increments, and set aside;
[0030] ③ Dissolve Poloxamer 188 in purified water, add manidipine hydrochloride and mix well, set aside;
[0031] ④ Add the mixture of lactose and hydroxypropyl methylcellulose into the high-efficiency wet granulator, stir and mix for 300 seconds, add the mixture ...
experiment example
[0032] Experimental example-clinical research of tablet of the present invention
[0033] In order to evaluate the effectiveness and safety of the Manidipine Hydrochloride Tablets of the present invention in treating mild to moderate essential hypertension, a randomized, double-blind, double-dummy, parallel-controlled multicenter phase II clinical study of Amlodipine Tablets was carried out. The results reported below.
[0034] 1. Clinical effectiveness research
[0035] A total of 238 cases were randomly enrolled in the test, of which 119 cases were enrolled in the test group, 11 cases were dropped out, and 108 cases were completed without elimination; 119 cases were enrolled in the control group, 7 cases were dropped out, 1 case was eliminated, and 111 cases were completed. Test group: half a tablet of manidipine hydrochloride (10 mg, Example 1) + 1 tablet of amlodipine analog tablet; the dosage of the drug was doubled for patients with unsatisfactory antihypertensive effec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com