Human monoclonal antibodies specific for glypican-3 and use thereof
A human monoclonal antibody, phosphatidylinositol technology, applied in the direction of antibodies, immunoglobulins, antibody medical components, etc., can solve the problem that the molecular mechanism of tumor metastasis is poorly understood.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
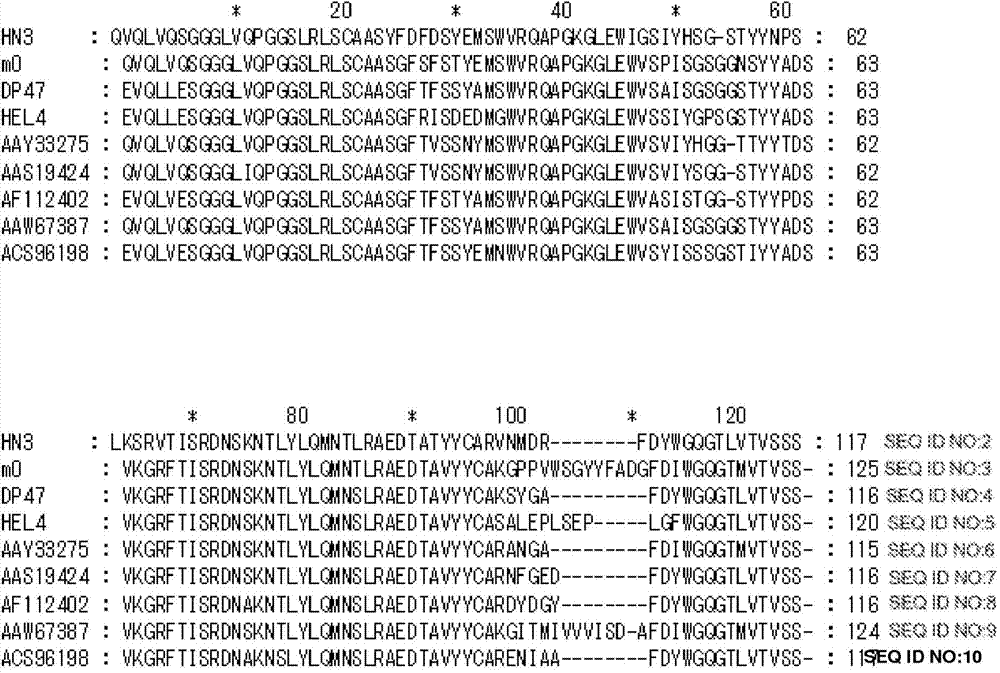
[0315] Example 1: HN3—a human single-domain monoclonal antibody that binds cell surface-associated Glypican 3 and inhibits HCC cell proliferation
[0316] This example describes the generation and characterization of high affinity single domain mAbs against tumor-associated GPC3.
[0317] A. Materials and methods
[0318] cell line
[0319] Human hepatoma cell lines HepG2, Hep3B, HuH-1, HuH-4, HuH-7 and SK-Hep1 were maintained in adherent monolayer cultures supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 1% L-glutamine and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) in D-MEM medium (Invitrogen, Carlsbad, CA) at 37 °C in 5% CO 2 incubate in air. Cells were harvested and the medium was changed twice a week. Cells were confirmed to be mycoplasma negative. The GPC3-negative cell line A431 (a human epithelial carcinoma cell line) was engineered to express high levels of GPC3 by transfection with a plasmid encoding GPC3. Both A431 and a stably transfec...
Embodiment 2
[0355] Example 2: HS20—a human monoclonal antibody that binds heparan sulfate chains on GPC3 and inhibits the migration of hepatocellular carcinoma cells
[0356] This example describes the generation and characterization of human mAbs that bind heparan sulfate on GPC3.
[0357] A. Materials and methods
[0358] cell line
[0359] A panel of six human HCC cell lines (SK-Hep1, HepG2, Hep3B, Huh-1, Huh-4, and Huh-7) was obtained from the Human Carcinogenesis Laboratory, National Cancer Institute (NCI), Bethesda, MD (National Cancer Institute (NCI) Laboratory of Human Carcinogenesis, Bethesda, Maryland). A431 (human epithelial carcinoma cell line) was obtained from the American Type Culture Collection (ATCC; Manassas, VA). The cell lines were cultured in RPMI or DMEM supplemented with 10% fetal bovine serum, 100 U / mL penicillin, 0.1 mg / mL streptomycin, and 2 mmol / L L-glutamine. Cells were harvested and the medium was changed twice a week. Cells were confirmed to be mycoplasm...
Embodiment 3
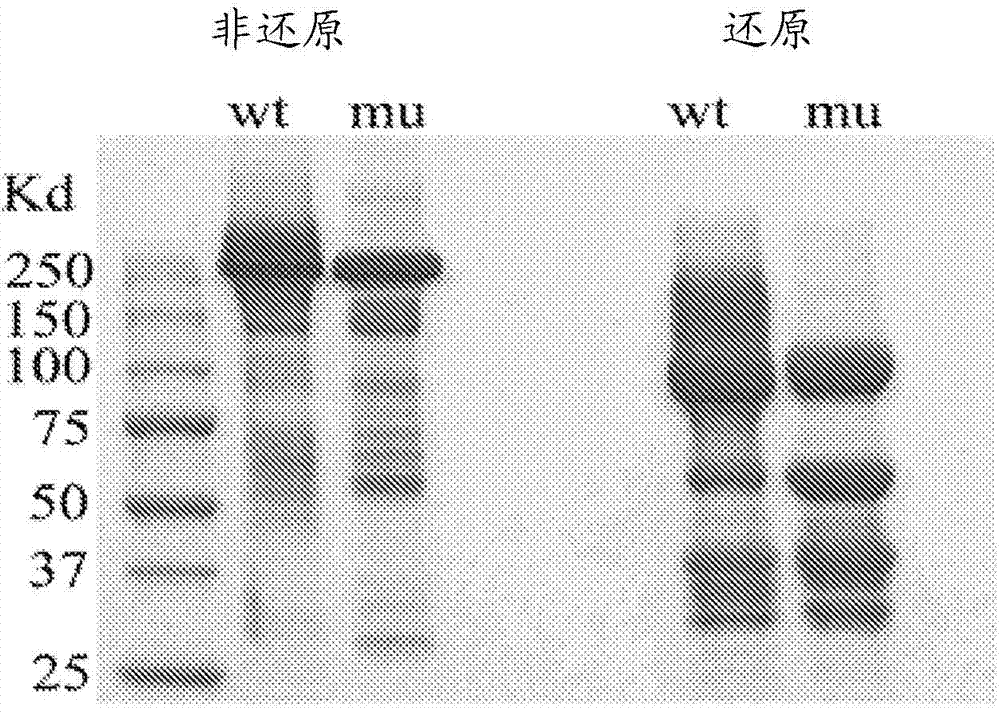
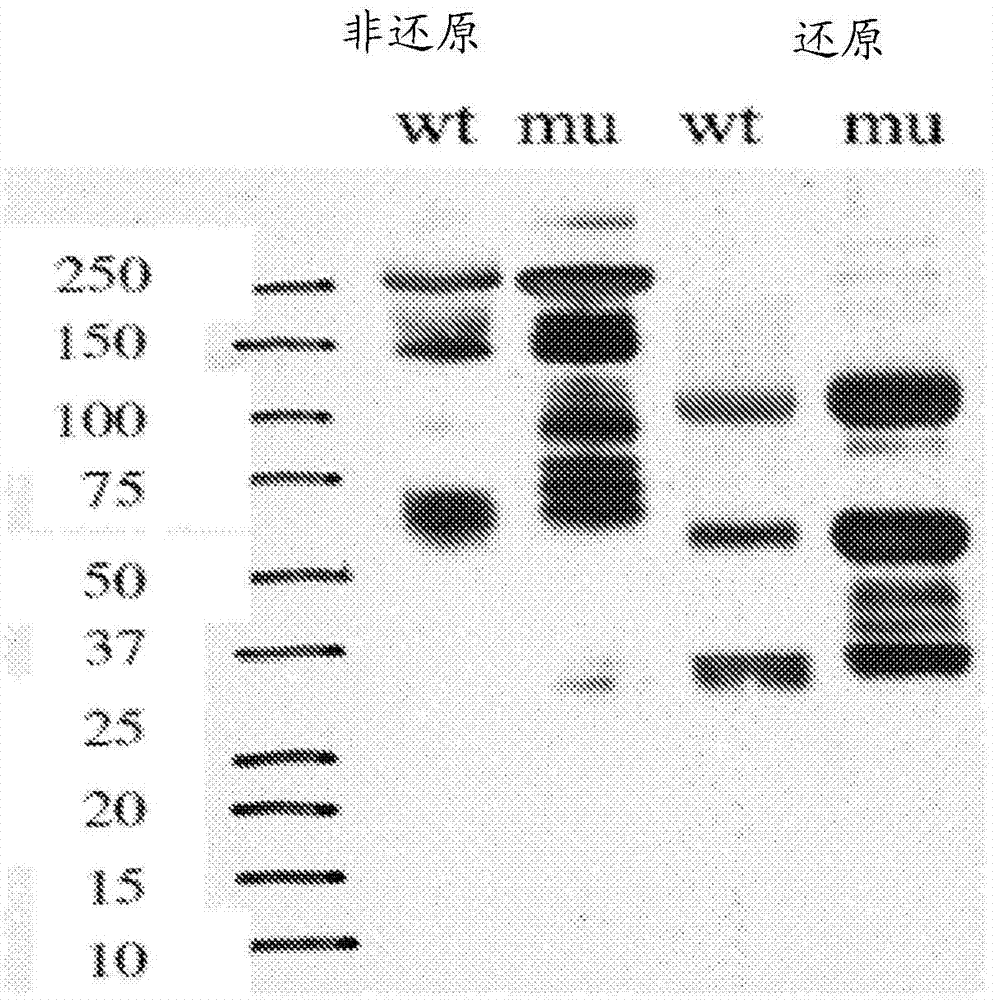
[0402] Example 3: Mutation of HS20 to remove N-glycosylation sites does not alter affinity and specificity
[0403] This example describes the modification of the H220 mAb to remove the N-glycosylation sites identified in the VL domain.
[0404] Two light chain bands were observed in HS20 IgG expressed in a HEK-293-based mammalian expression system, suggesting the presence of N-glycosylation sites. By analyzing the sequence of the light chain, a possible N-glycosylation site was identified in CDR2 of the VL domain of HS20 (amino acid residue 50 in SEQ ID NO: 16). The presence of N-glycosylation sites produced an additional band of approximately 30 kDa when analyzed by SDS-PAGE; however, when purified HS20 IgG was treated with the endoglycosidase PNGase to remove all N-glycosylation , a single band was observed (Fig. 15A). Western blot of H2S0 mAb treated with PNGase also demonstrated a single VL band, whereas untreated antibody produced two bands of the VL domain (Fig. 15B)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com