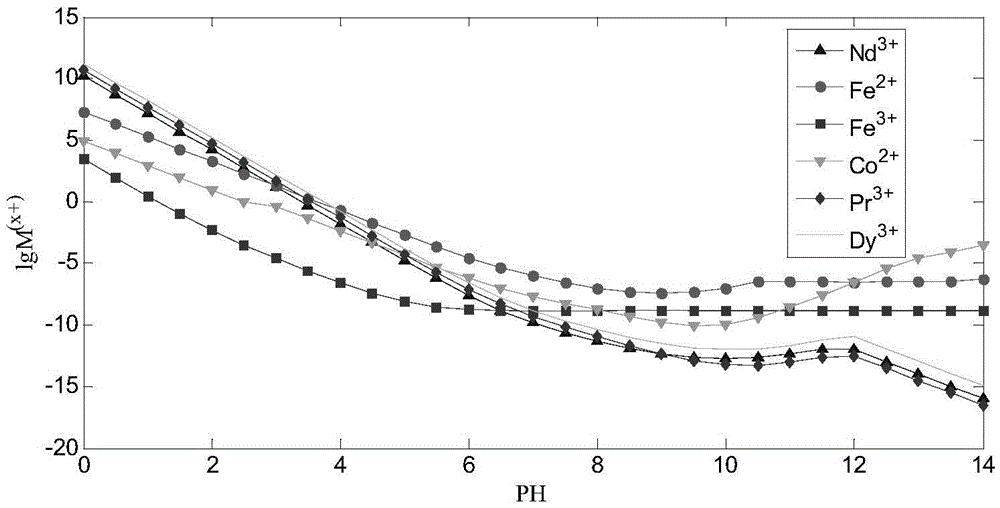
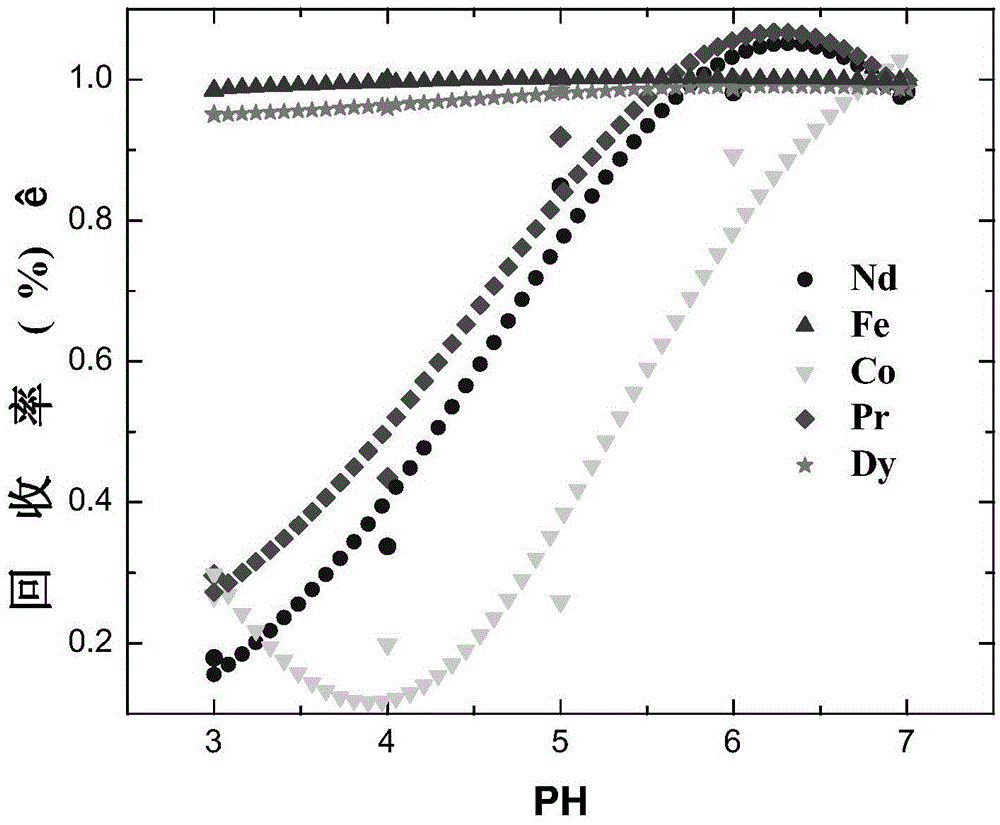
A method for simultaneously recovering neodymium, praseodymium, dysprosium, cobalt, and iron from NdFeB sludge under CO3-OH system
A CO3-OH, NdFeB technology, applied in the field of praseodymium, cobalt, dysprosium, iron, and extraction of neodymium, can solve the problems of waste of rare earth resources and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Table 1 "CO 3 The main chemical reactions and equilibrium constants involved in the -OH" system
[0072] No.
Reactions
logK
No.
Reactions
logK
1
h 2 O=H + +OH —
logK w =-14
16
Nd+OH — =Nd(OH) 2+
logK nh =5.5
2
co 2+ +OH — =Co(OH) +
logK ch1 =3.3
17
Pr+OH — =Pr(OH) 2+
logK pH =4.3
3
co 2+ +2OH — =Co(OH) 2 0
logK ch2 =9.2
18
Dy+OH — =Dy(OH) 2+
logK dh =5.2
4
co 2+ +3OH — =Co(OH) 3 —
logK ch3 =10.5
19
Co(OH) 2 (s)=Co 2+ +2OH —
logKspch=-14.23
5
co 2+ +4OH — =Co(OH) 4 2-
logK ch4 =10.2
20
Fe(OH) 2 (s)=Fe 2+ +2OH —
logKspf2h=-16.31
6
2Co 2+ +OH — =Co 2 (OH) 3+
logK ch21 =2.7
21
Fe(OH) 3 (s)=Fe 3+ +3OH —
logKspf3h=-38.55
7
4Co 2+ +4OH — =Co 4 (OH) 4 4+
logK ch44 =25.6
2...
Embodiment 2
[0127] Table 1 "CO 3 The main chemical reactions and equilibrium constants involved in the -OH" system
[0128] No.
Reactions
logK
No.
Reactions
logK
1
h 2 O=H + +OH —
logK w =-14
15
Nd+OH — =Nd(OH) 2+
logK nh =5.5
2
co 2+ +OH — =Co(OH) +
logK ch1 =3.3
16
Pr+OH — =Pr(OH) 2+
logK pH =4.3
[0129] 3
co 2+ +2OH — =Co(OH) 2 0
logK ch2 =9.2
17
Dy+OH — =Dy(OH) 2+
logK dh =5.2
4
co 2+ +3OH — =Co(OH) 3 —
logK ch3 =10.5
18
Co(OH) 2 (s)=Co 2+ +2OH —
logKspch=-14.23
5
co 2+ +4OH — =Co(OH) 4 2-
logK ch4 =10.2
19
Fe(OH) 2 (s)=Fe 2+ +2OH —
logKspf2h=-16.31
6
2Co 2+ +OH — =Co 2 (OH) 3+
logK ch21 =2.7
20
Fe(OH) 3 (s)=Fe 3+ +3OH —
logKspf3h=-38.55
6 --> 7
4Co 2+ +4OH — =Co 4 (OH) 4 4+
...
Embodiment 3
[0133] Table 1 "CO 3 The main chemical reactions and equilibrium constants involved in the -OH" system
[0134] No.
Reactions
logK
No.
Reactions
logK
1
h 2 O=H + +OH —
logK w =-14
16
Nd+OH — =Nd(OH) 2+
logK nh =5.5
2
co 2+ +OH — =Co(OH) +
logK ch1 =3.3
17
Pr+OH — =Pr(OH) 2+
logK p H=4.3
3
co 2+ +2OH — =Co(OH) 2 0
logK ch2 =9.2
18
Dy+OH — =Dy(OH) 2+
logK dh =5.2
4
co 2+ +3OH — =Co(OH) 3 —
logK ch3 =10.5
19
Co(OH) 2 (s)=Co 2+ +2OH —
logKspch=-14.23
5
co 2+ +4OH — =Co(OH) 4 2-
logK ch4 =10.2
20
Fe(OH) 2 (s)=Fe 2+ +2OH —
logKspf2h=-16.31
6
2Co 2+ +OH — =Co 2 (OH) 3+
logK ch21 =2.7
21
Fe(OH) 3 (s)=Fe 3+ +3OH —
logKspf3h=-38.55
7
4Co 2+ +4OH — =Co 4 (OH) 4 4+
logK ch44 =25.6
2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com