A method for simultaneously recovering neodymium, praseodymium, dysprosium, cobalt, and iron from NdFeB sludge under nh3-oh system
A technology of NH3-OH and NdFeB, which is applied in the fields of cobalt, praseodymium, iron, dysprosium, and neodymium extraction, which can solve problems such as environmental pollution and waste of rare earth resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
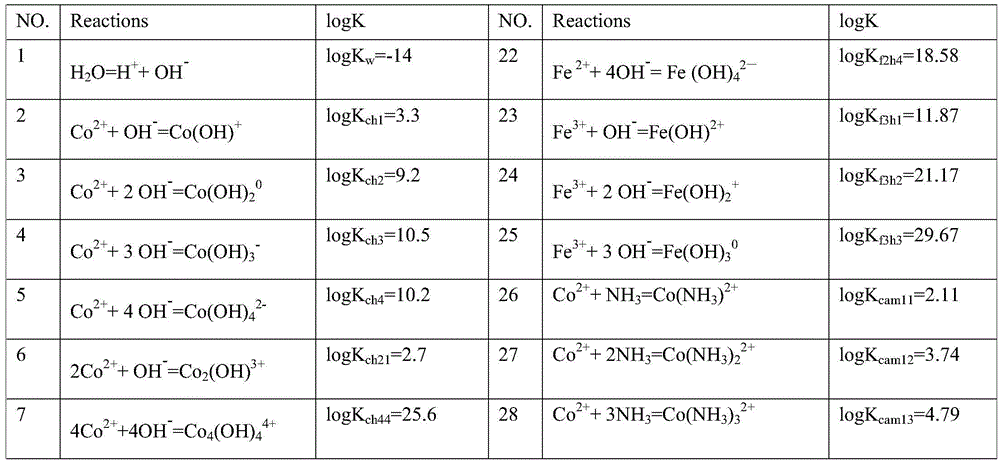
[0062] Table 1 "NH 3 The main chemical reactions and equilibrium constants involved in the -OH" system
[0063] NO.
Reactions
logK
NO.
Reactions
logK
1
H 2 O=H + +OH —
logK w =-14
22
Fe 2+ +4OH — =Fe(OH) 4 2-
logK f2h4 =18.58
2
Co 2+ +OH — =Co(OH) +
logK ch1 =3.3
23
Fe 3+ +OH — =Fe(OH) 2+
logK f3h1 =11.87
3
Co 2+ +2OH — =Co(OH) 2 0
logK ch2 =9.2
24
Fe 3+ +2OH — =Fe(OH) 2 +
logK f3h2 =21.17
4
Co 2+ +3OH — =Co(OH) 3 -
logK ch3 =10.5
25
Fe 3+ +3OH — =Fe(OH) 3 0
logK f3h3 =29.67
5
Co 2+ +4OH — =Co(OH) 4 2 -
logK ch4 =10.2
26
Co 2+ +NH 3 =Co(NH 3 ) 2+
logK cam11 =2.11
6
2Co 2+ +OH — =Co 2 (OH) 3+
logK ch21 =2.7
27
Co 2+ +2NH 3 =Co(NH 3 ) 2 2+
logK cam12 =3.74
7
4Co 2+ +4OH — =Co 4 (OH) 4 4+
logK ch44 =25.6
28
Co 2+ +3NH 3 =Co(NH 3 ) 3 2+
logK cam13 =4.79
8
Fe 2+ +OH — =Fe(OH) +
logK f2h1 =5.56
29
Co 2+ +4NH 3 =Co(NH 3 ) 4 2+
logK cam14 =5.55
9
Fe 2+ +2OH — =Fe(OH) 2 0
logK f2h2 =9.77
30
Co 2+ +5NH 3 =Co(NH...
Embodiment 2
[0110] Table 2 "NH 3 The main chemical reactions and equilibrium constants involved in the -OH" system
[0111] NO.
Reactions
logK
NO.
Reactions
logK
1
H 2 O=H + +OH —
logK w =-14
22
Fe 2+ +4OH — =Fe(OH) 4 2-
logK f2h4 =18.58
2
Co 2+ +OH — =Co(OH) +
logK ch1 =3.3
23
Fe 3+ +OH — =Fe(OH) 2+
logK f3h1 =11.87
3
Co 2+ +2OH — =Co(OH) 2 0
logK ch2 =9.2
24
Fe 3+ +2OH — =Fe(OH) 2 +
logK f3h2 =21.17
4
Co 2+ +3OH — =Co(OH) 3 -
logK ch3 =10.5
25
Fe 3+ +3OH — =Fe(OH) 3 0
logK f3h3 =29.67
6 --> 5
Co 2+ +4OH — =Co(OH) 4 2 -
logK ch4 =10.2
26
Co 2+ +NH 3 =Co(NH 3 ) 2+
logK cam11 =2.11
6
2Co 2+ +OH — =Co 2 (OH) 3+
logK ch21 =2.7
27
Co 2+ +2NH 3 =Co(NH 3 ) 2 2+
logK cam12 =3.74
7
4Co 2+ +4OH — =Co 4 (OH) 4 4+
logK ch44 =25.6
28
Co 2+ +3NH 3 =Co(NH 3 ) 3 2+
logK cam13 =4.79
8
Fe 2+ +OH — =Fe(OH) +
logK f2h1 =5.56
29
Co 2+ +4NH 3 =Co(NH 3 ) 4 2+
logK cam14 =5.55
9
Fe 2+ +2OH — =Fe(OH) 2 0
logK f2h2 =9.77
30
Co 2+ +5NH 3...
Embodiment 3
[0115] Table 3 "NH 3 The main chemical reactions and equilibrium constants involved in the -OH" system
[0116]
[0117]
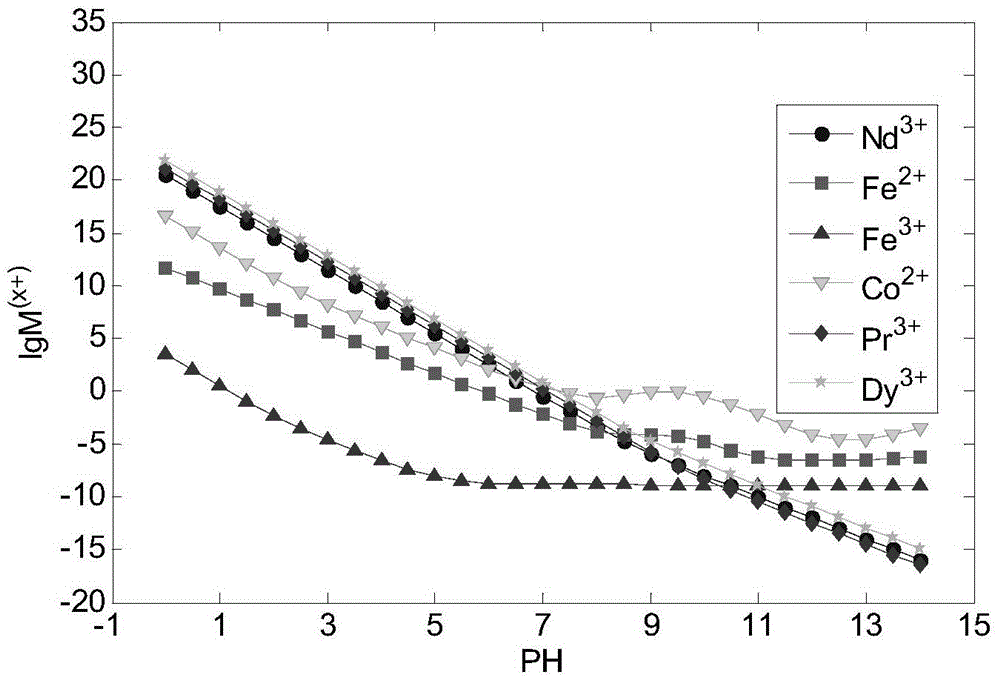
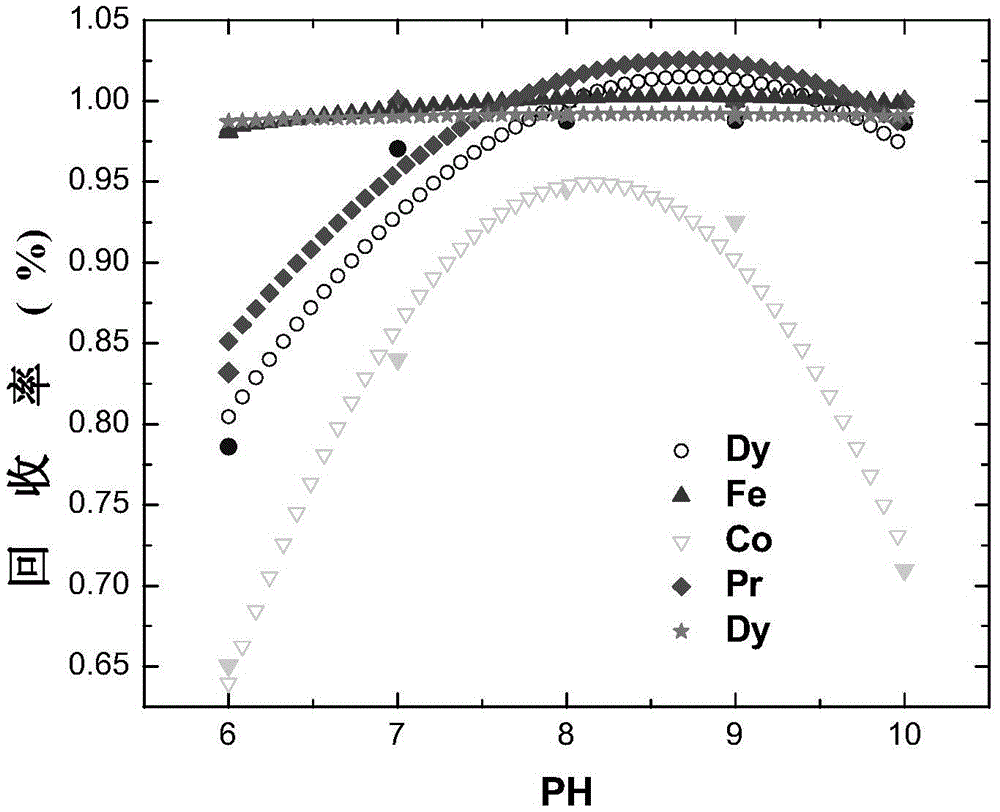
[0118] In the theoretical part: First, check the OH-NH 3 The possible reactions of neodymium, praseodymium, dysprosium, cobalt, and iron in the system and the equilibrium constants of each reaction are shown in Table 1. Based on OH-NH through chemical balance, mass balance, and charge conservation 3 The thermodynamic model under the system can be programmed by MATLAB to get the values of [Nd], [Pr], [Dy], [Fe2], [Fe3], [Co] at different pH values, so as to establish a thermodynamic model, such as figure 1 Shown. according to figure 1 It can be considered that trivalent iron ions should be selected as much as possible, and the pH range of the optimal precipitation of neodymium, praseodymium, dysprosium, cobalt, and iron should be within 6-10, and the combined precipitation of neodymium, praseodymium, dysprosium, cobalt, and iron can be obtained through a on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com