Gastrodin drugs and their compositions and uses
A technology of acetylgastrodin and crystalline hydrate, which is applied in the field of medicine, can solve problems such as unpublished literature reports, and achieve the effects of improving hygroscopicity, improving operability, and facilitating storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
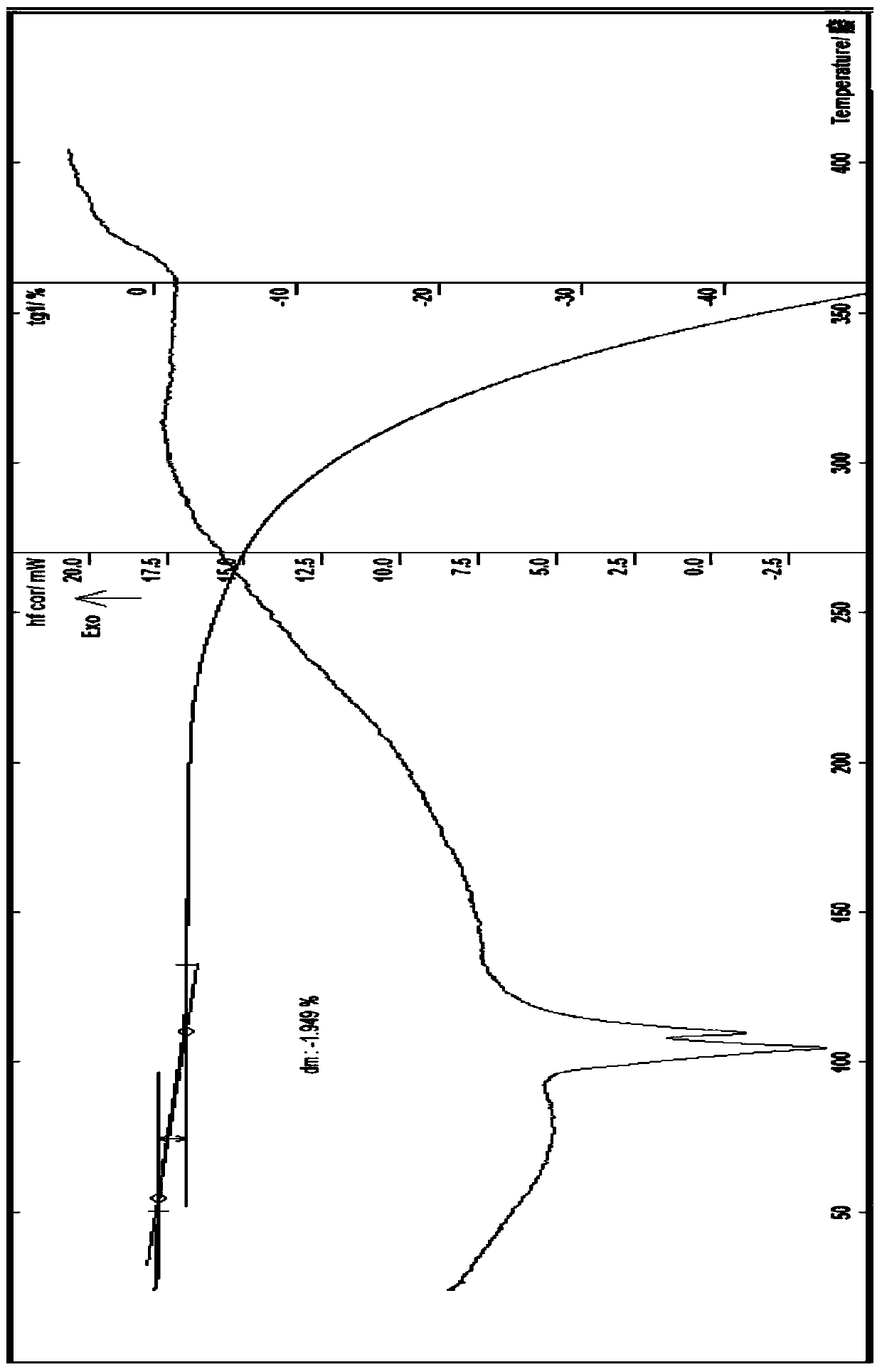
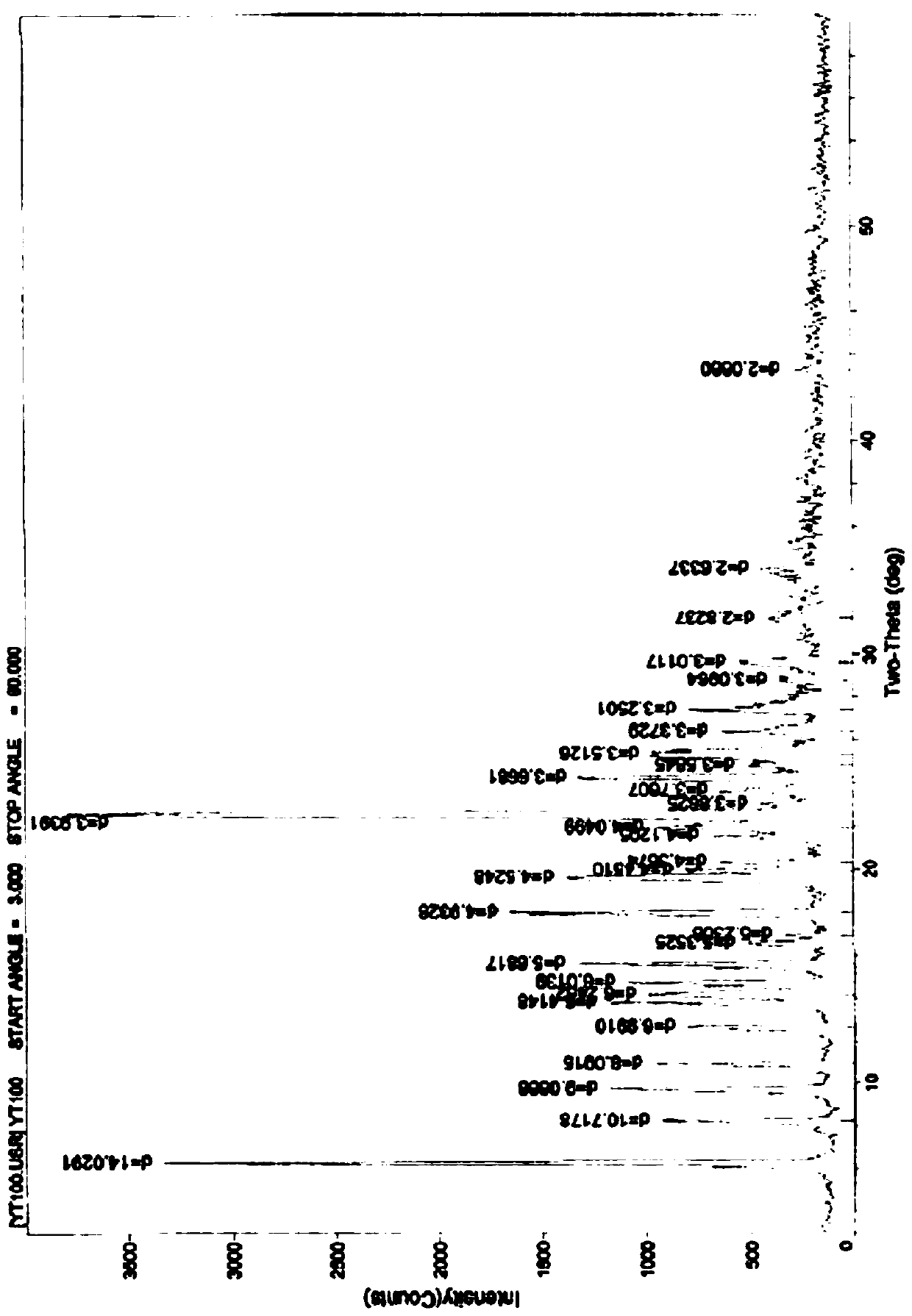
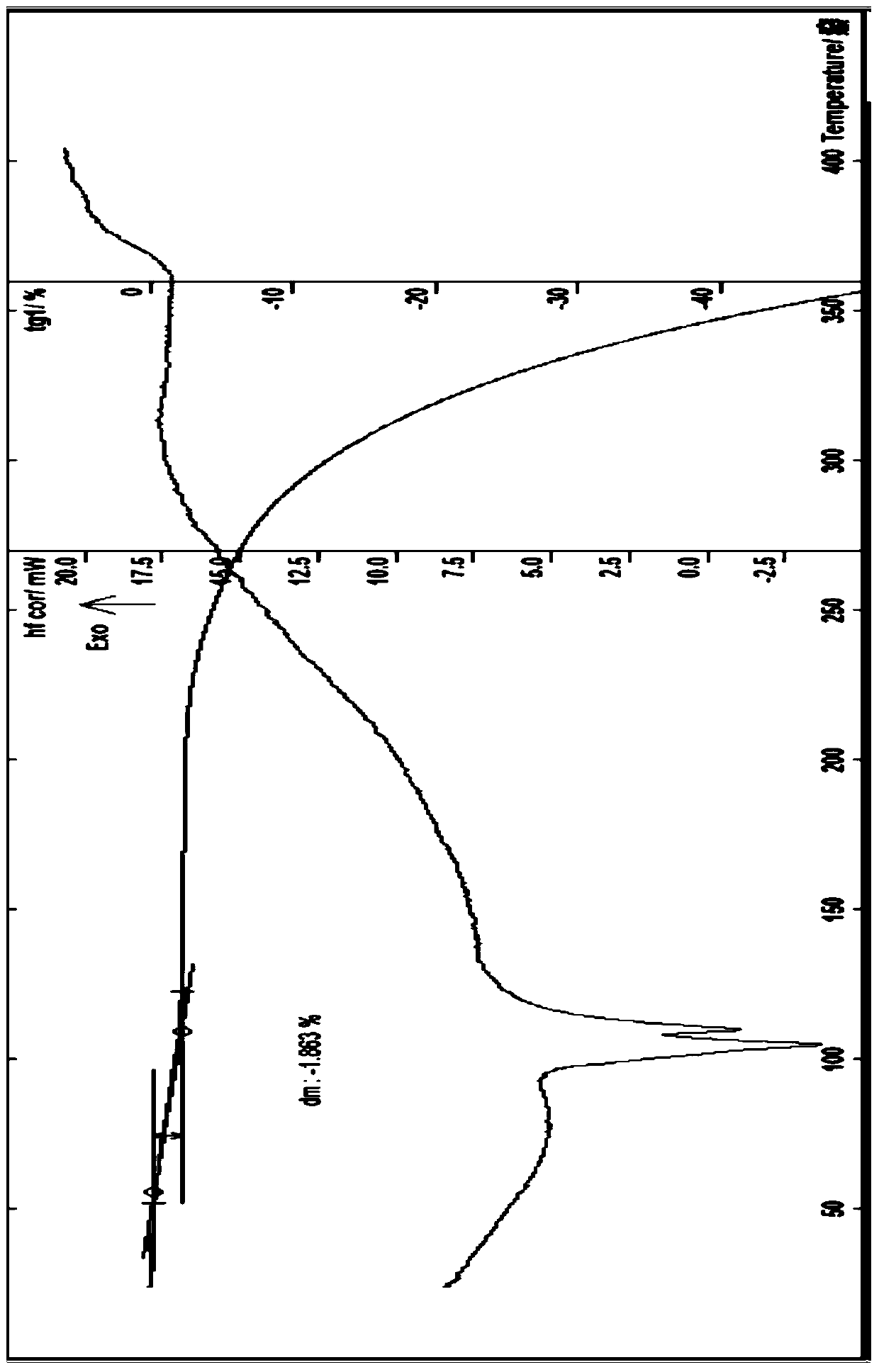
[0069] Embodiment 1 (attached figure 2 ), about 6.29, 8.24, 9.75, 10.93, 12.65, 13.79, 14.17, 14.72, 15.58, 16.55, 16.94, 17.97, 19.60, 19.93, 20.32, 21.55, 21.92, 22.55, 23.00, 243.6 , 24.82, 25.33, 26.40, 27.42, 28.81, 29.64, 31.66, 34.01, etc. have characteristic peaks.
[0070] In another embodiment (implementation 2) (attached Figure 4 ), measured by powder X-ray diffraction method, within the measurement range of diffraction angle 2θ (3-60°), the acetylgastrodin 0.5 hydrate of the present invention can have corresponding characteristic values at positions including the following 2θ values, about 6.30, 8.33,9.80,11.00,12.59,13.84,14.21,14.78,15.61,16.64,17.08,18.04,19.65,20.02,20.36,21.61,21.96,22.60,23.70,24.30,25.27,26.53,27.66,28.58,29.65,31.75, There are characteristic peaks such as 34.43.
Embodiment 2
[0072] Example 2 Preparation of Acetyl Gastrodin 0.5 Hydrate In a three-necked flask, add 230ml of distilled THF, 20ml of methanol, 4-formylphenyl-2',3',4',6'-tetraacetyl-β -D-Glucopyranoside 10g, stir to dissolve, add 0.5g of sodium borohydride, 1g of potassium borohydride, stir, control the temperature between about 5-40°C, stir, monitor the reaction process with thin layer chromatography (TLC) After the reaction is completed, mix the reactant with an appropriate amount of ice water, control the pH of the solution to about 6.5-7.0, continue to stir for about 30 minutes, extract with chloroform, concentrate the chloroform extract to dryness, and add an appropriate amount of methanol to heat the obtained solid to dissolve it. Add an appropriate amount of activated carbon, stir for about 30 minutes, filter, add an appropriate amount of water to the filtrate until the solution is nearly muddy, let it cool, and fully separate out the solid crystals, filter, and recrystallize the o...
Embodiment 3
[0073] Example 3 Preparation of acetylgastrodin 0.5 hydrate In a three-necked flask, add isopropanol 1000ml, methanol 30ml, 4-formylphenyl-2',3',4',6'-tetraacetyl-β- D-Glucopyranoside 10g, stir to dissolve, add 3g of sodium borohydride, stir, control the temperature at about 0-30°C, stir, monitor the reaction progress with thin layer chromatography (TLC), after the reaction is completed, the reactant Mix with an appropriate amount of ice water, control the solution pH=6.5-7.0, continue stirring for about 30 minutes, extract with chloroform, concentrate the chloroform extract to dryness, add ethyl acetate to dissolve, add appropriate amount of activated carbon, stir for about 30 minutes, filter , Concentrate the obtained filtrate to dryness, add an appropriate amount of methanol to heat to dissolve, add an appropriate amount of water, cool, leave to precipitate the solid crystallization completely, filter, and recrystallize the obtained solid twice with an appropriate amount of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com