A kind of polyaromatic ester containing phenothiazine and its preparation method and application
A polyarylester and phenothiazine technology, applied in the field of polyarylester and its preparation, can solve the problems of poor thermal stability, low polyester solubility, difficult to process into a film, etc., and achieve good high temperature resistance and high thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
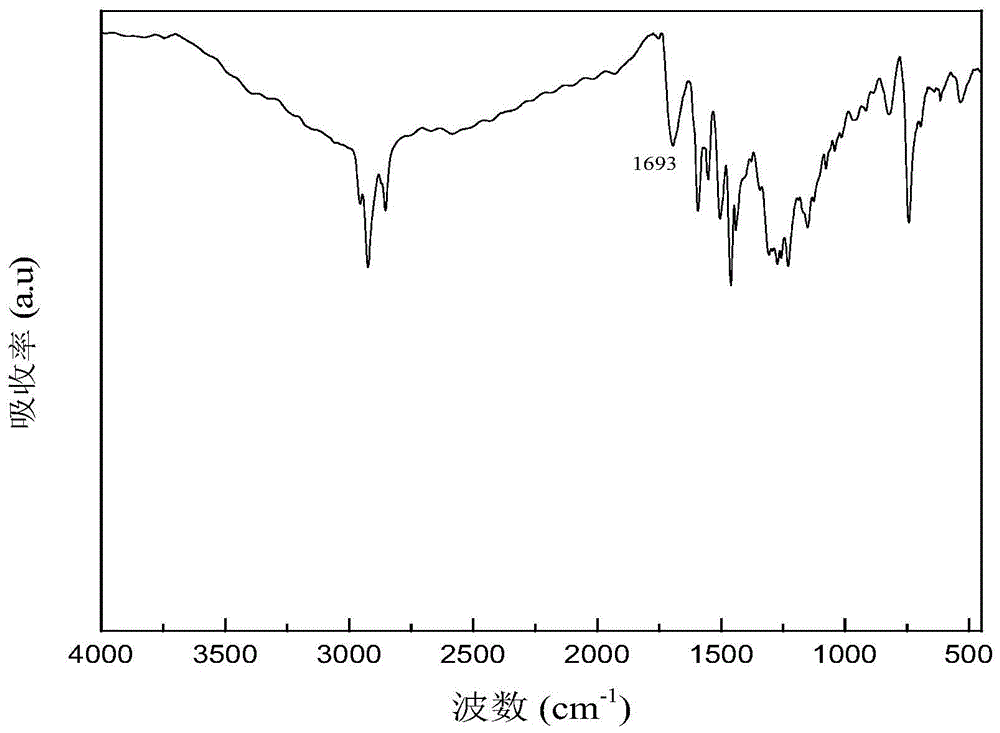
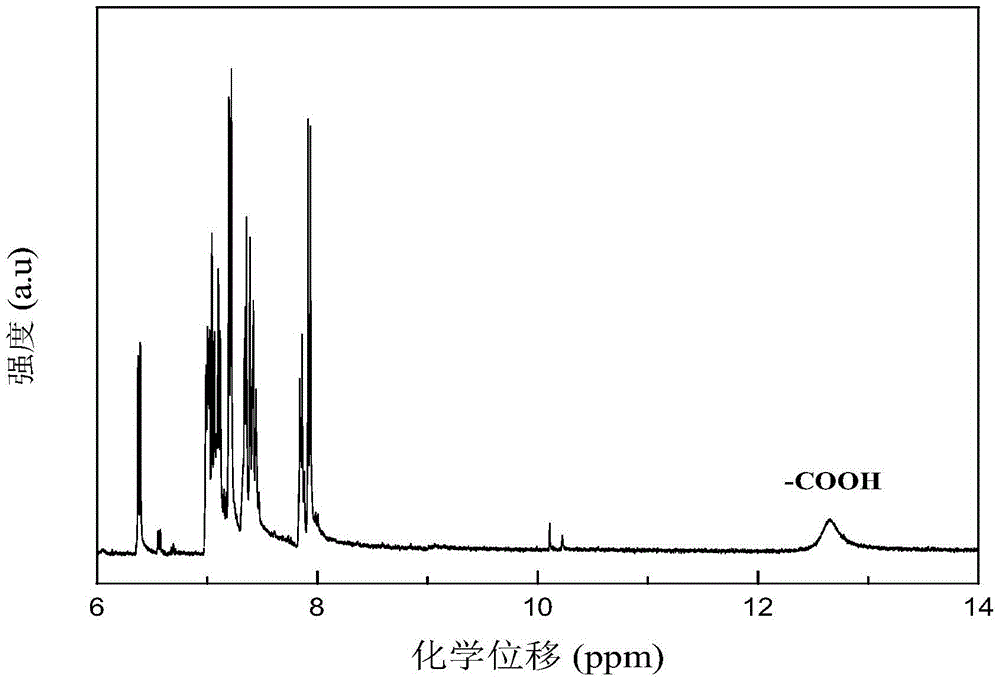
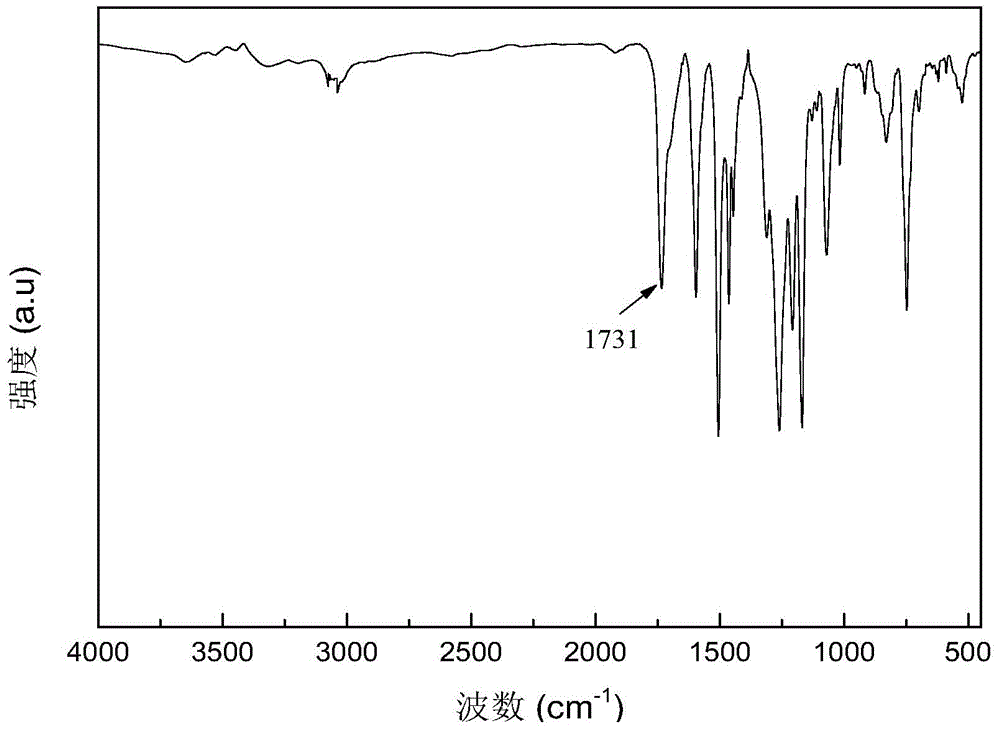
[0059] Specific implementation mode one: the present implementation mode is a polyarylate containing phenothiazine whose structural formula is The Ar is The value range of n is 1≤n≤100, and n is an integer.
[0060] The advantage of this implementation mode:
[0061] 1. The phenothiazine-containing polyarylate prepared in this embodiment overcomes the disadvantage that polyarylate was difficult to dissolve in general organic solvents, while retaining its high thermal stability; the phenothiazine-containing polyarylate utilizes The external phenothiazine group reduces the degree of conjugation of the entire main chain to increase its solubility, making polyarylate soluble in most organic solvents; while the internal phenothiazine group has electron-rich sulfur and The nitrogen heterocyclic structure increases the distribution of the electron cloud, making the polymer prone to light absorption, and causes color changes after oxidation, and can undergo reversible changes ...
specific Embodiment approach 2
[0066] Specific embodiment two: this embodiment is a kind of preparation method of the polyarylate containing phenothiazine is finished according to the following steps:
[0067] One, synthetic N-(4-nitrophenyl) phenothiazine: NaH is dissolved in the organic solvent, obtains NaH solution; Add phenothiazine and p-fluoronitrobenzene in the NaH solution, then in nitrogen atmosphere and React at a temperature of 120°C to 140°C for 16h to 20h, then use distilled water to precipitate the reactant A, and then use absolute ethanol to recrystallize the reactant A to obtain N-(4-nitrophenyl)phenothiene Zinc;
[0068] The volume ratio of the mass of NaH described in step 1 to the organic solvent is (0.05g~0.2g): 10mL;
[0069] The mol ratio of phenothiazine described in step 1 and p-fluoronitrobenzene is 1:1;
[0070] The volume ratio of the amount of the substance of the phenothiazine described in step 1 to the organic solvent is (0.1mol~0.5mol): 150mL;
[0071] 2. Synthesis of N-(4-...
specific Embodiment approach 3
[0096] Specific embodiment three: the difference between this embodiment and specific embodiment two is: the monomer containing dihydroxy described in step five ② is Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com