Combined therapy composition and combined therapy method for treating cancers
一种免疫治疗、靶向治疗剂的技术,应用在化学仪器和方法、药物组合、基因治疗等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0468] Enrichment of Human Dendritic Cells (DC) from PBMC
[0469] Human PBMCs were prepared from erythrocytes obtained from healthy voluntary donors by Ficoll centrifugation. Dendritic cells were enriched from magnetic beads (Miltenyi Biotec Inc. San Diego, CA) with a mixture of anti-CD3, anti-CD19, anti-CD20, anti-CD14 and anti-CD16 antibodies from human PBMCs by using negative subtraction. Enriched DCs were stained with goat anti-mouse FITC (lineage), HLA-DR-APCCy7, CD123-BV421 and CD11C-APC. Stained cells were analyzed on BDLSR Fortessa (BD Biosciences). Anti-CD3 mAb, anti-CD4 mAb, anti-CD11C mAb, anti-CD19 mAb, anti-CD14 mAb, anti-CD16 mAb, anti-CD123 mAb were purchased from BD Biosciences, CA or Biolegend, San Diego , CA.
[0470] Stimulation of enriched human DCs and expression of cytokines
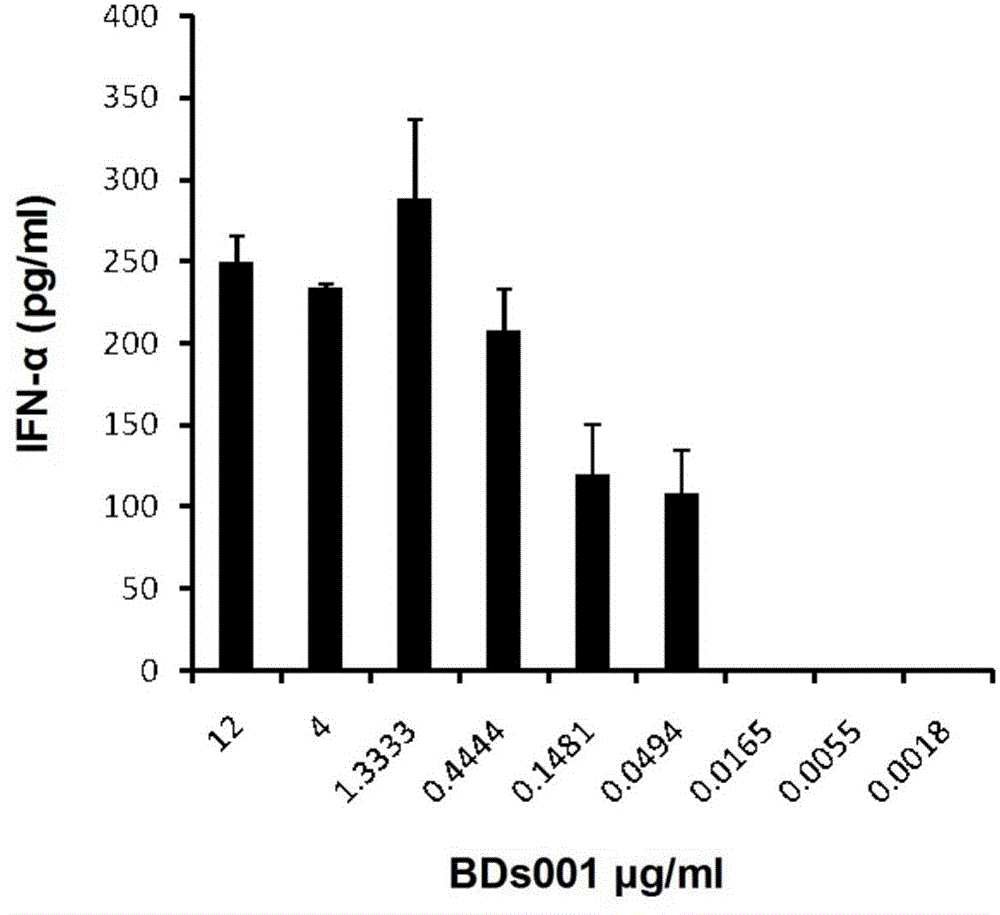
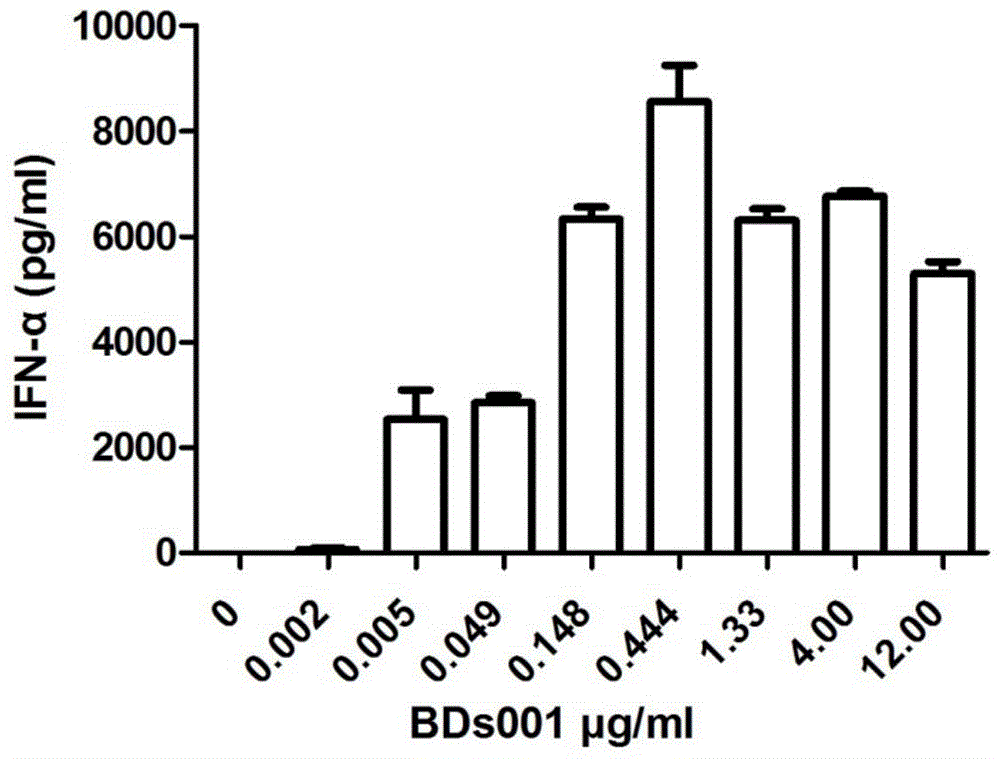
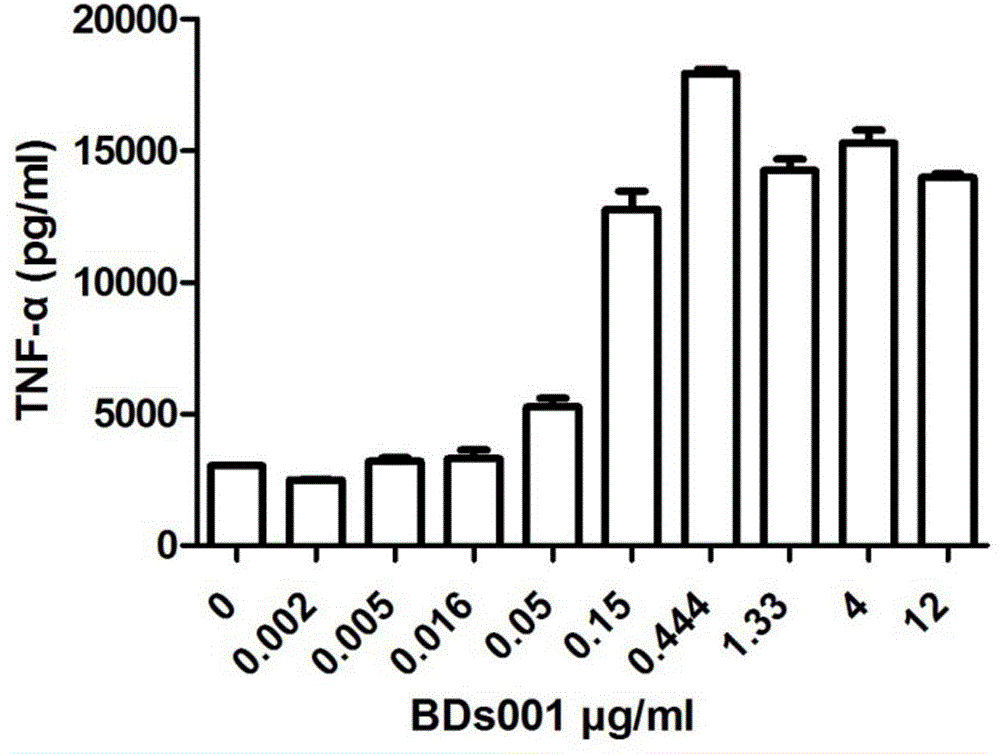
[0471] Will be 1-2 x 10 5 Enriched DCs were seeded in 96-well plates in 100 μl of medium, 100 μl of diluted stimulators including BDs100 (resiquimod) were added to the pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com