Combination Therapy For Treatment Of Disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
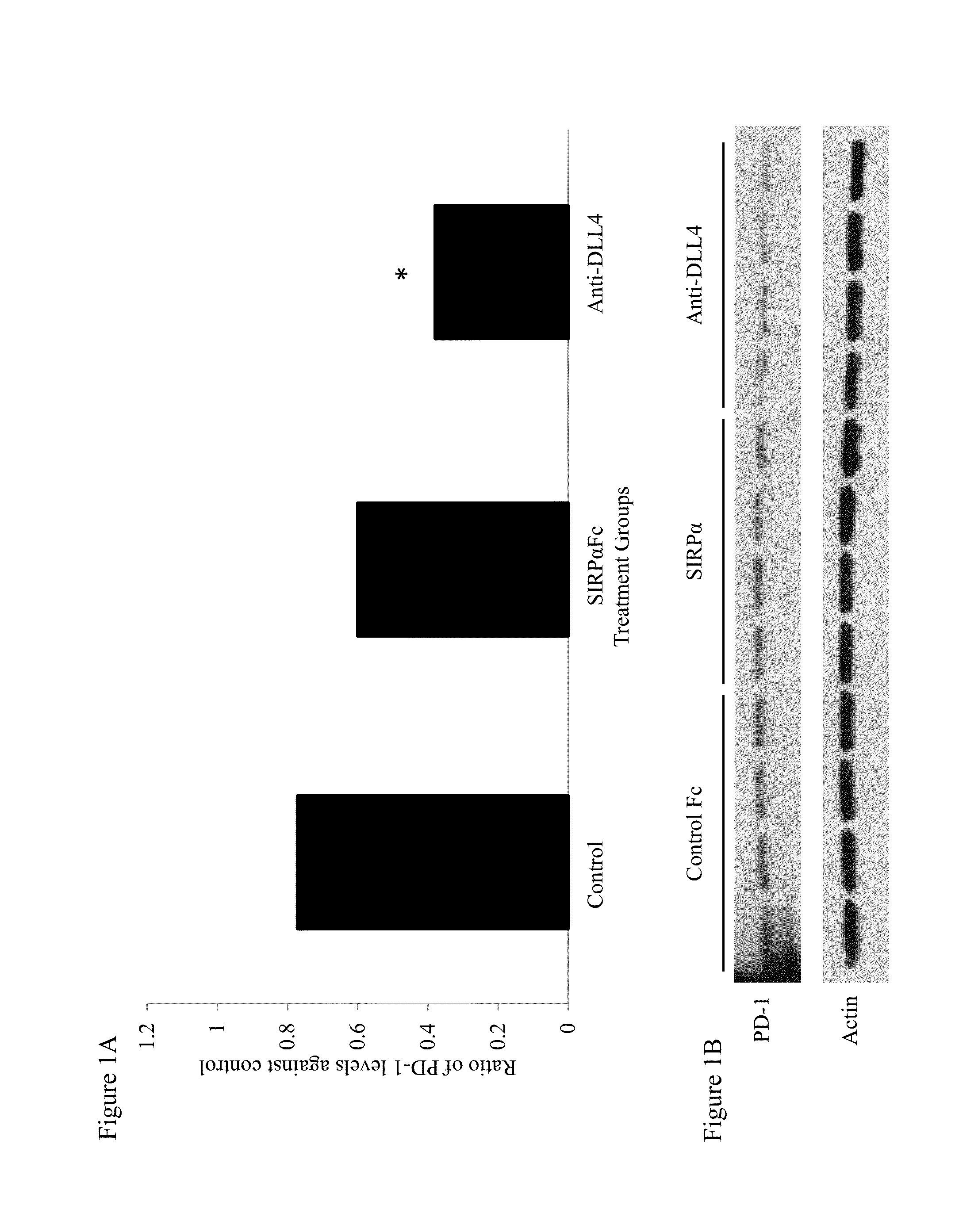
Effect of Anti-DLL4 Antibody on PD-1 Expression in Cells from CT26.WT Tumors
[0326]The murine colon carcinoma CT26.WT was obtained from ATCC. CT26.WT cells were injected subcutaneously into the rear flanks of 6-8 week old Balb / C mice. Tumors were allowed to grow until they reached an average size of 200 mm3. The mice were randomized into groups (n=10) and treated with anti-mouse DLL4 (mDLL4) antibody 21R30 (10 mg / kg, weekly), SIRPα-Fc protein (10 mg / kg, biweekly), or a control Fc protein (10 mg / kg, weekly). The agents were administered by injection into the intraperitoneal cavity. Fourteen days after the initial dose, the tumors were harvested and total protein was isolated from individual mice using tissue extraction buffer (Life Technologies). Proteins (20 μg) were resolved by SDS-PAGE gel electrophoresis, and Western blot analysis was performed using an anti-PD-1 antibody.
[0327]As shown in FIG. 1A, PD-1 expression in tumor cell lysates from mice treated with anti-mDLL4 antibody 21...
example 2
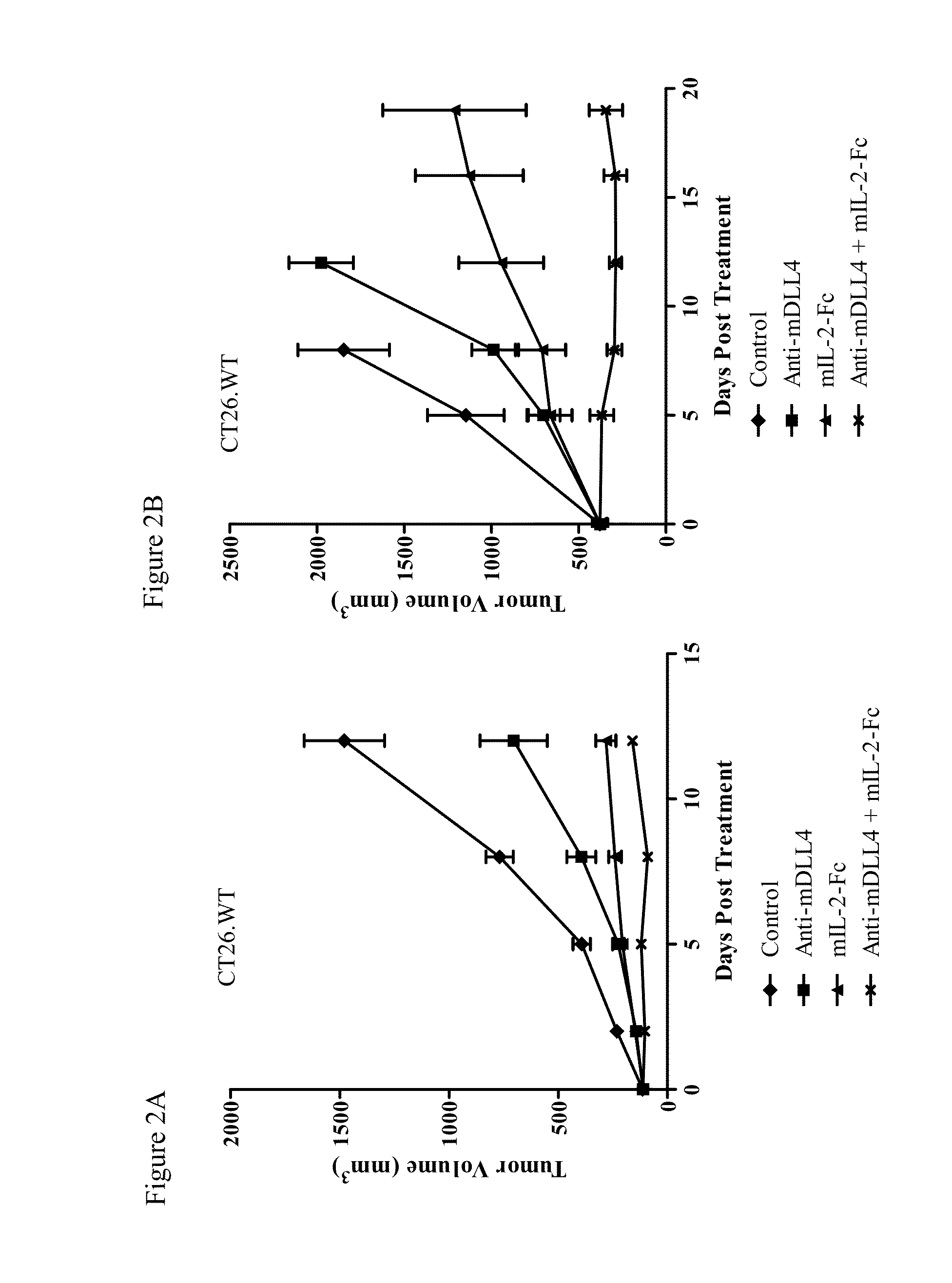
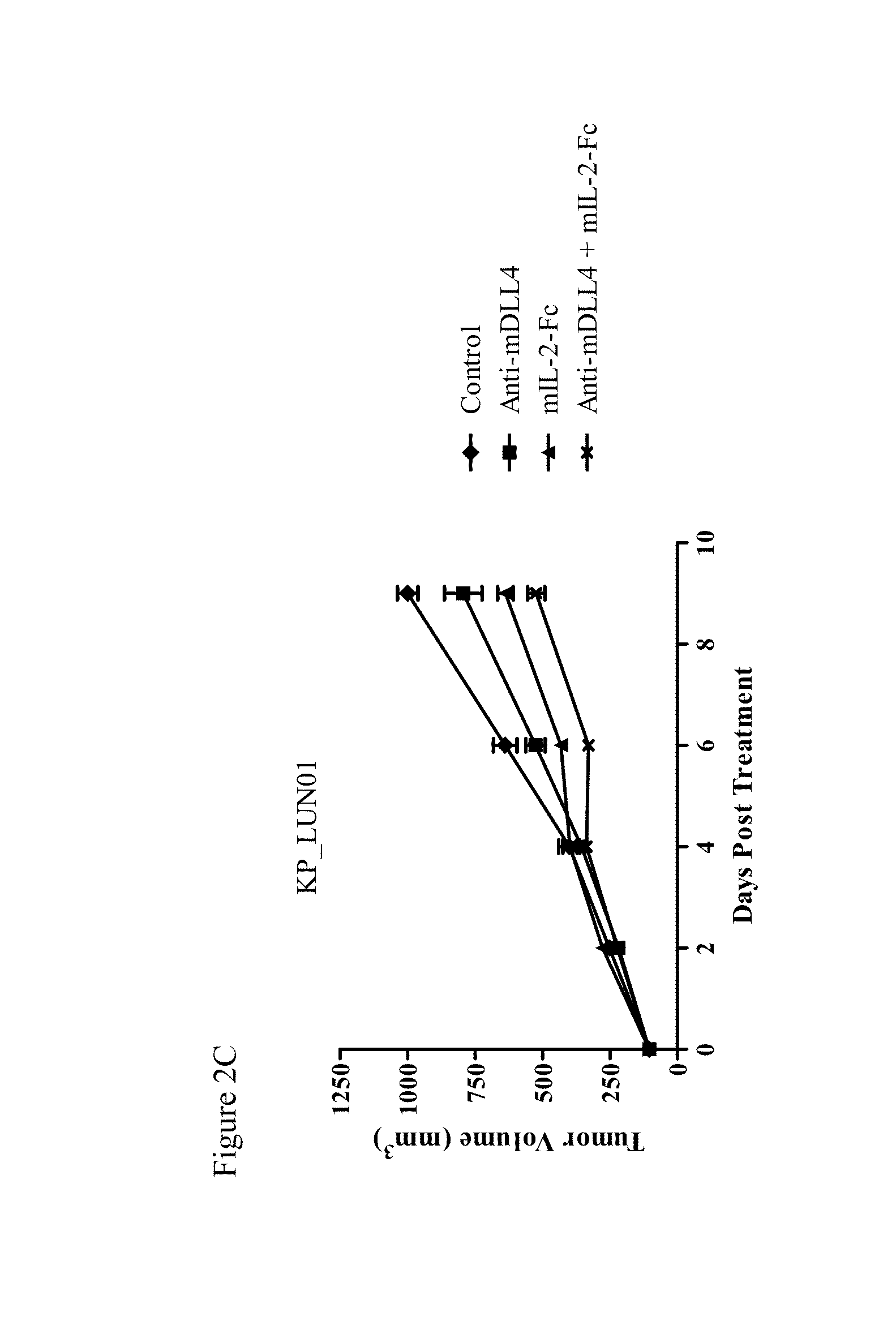
Effect of Mouse IL-2-Fc and Anti-mDLL4 Antibody on Tumor Growth
[0329]Murine colon carcinoma CT26.WT cells were injected subcutaneously into the flanks of 6-8 week old Balb / C mice. Tumors were allowed to grow to an average tumor volume of 100 mm3. The mice were randomized into groups (n=10) and treated with anti-mDLL4 antibody 21R30 (30 mg / kg, weekly), mIL-2-Fc protein (1 mg / kg, 5 days each week), or a combination of anti-mDLL4 antibody and mIL-2-Fc. An anti-GFP antibody and a murine Fc protein were used as controls for 21R30 and mIL-2-Fc, respectively. Agents were administered by intraperitoneal injection. Tumor growth was monitored and tumor volumes measured using electronic calipers at the indicated time points. The data are expressed as average tumor volume ±SEM.
[0330]As shown in FIG. 2A, anti-mDLL4 antibody 21R30 and mIL-2-Fc both inhibited CT26.WT tumor growth as single agents as compared to control. Furthermore, a combination of the anti-mDLL4 antibody and mIL-2-Fc inhibited t...
example 3
Cell Cytotoxicity Assays
[0337]For natural killer (NK) cytotoxicity assays, the mouse lymphoblast cell line YAC-1 and the mouse colon carcinoma cell line CT26.WT were cultured in RPMI 1640 culture medium (Gibco / Life Technologies, Grand Island, N.Y.) supplemented with 10% (v / v) fetal bovine serum (FBS), 2 mM L-glutamine, 100 U / ml penicillin, and 100 μg / ml streptomycin (Gibco) at 37° C. in a humidified atmosphere of 5% CO2. YAC-1 cells are known to be sensitive to NK cell activity and are a good target for NK cell assays.
[0338]Cells were harvested from the spleens of the CT26.WT tumor-bearing mice described above in Example 2. Cells were plated in 96-well V-bottom plates in RPMI 1640 culture medium (Gibco / Life Technologies, Grand Island, N.Y.) supplemented with 10% (v / v) fetal bovine serum (FBS), 2 mM L-glutamine, 100 U / ml penicillin, and 100 μg / ml streptomycin (Gibco). Target cells (YAC-1 or CT26.WT) were labeled with 10 μM calcein AM (Life Technologies) for 1 hour at 37° C. and then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com