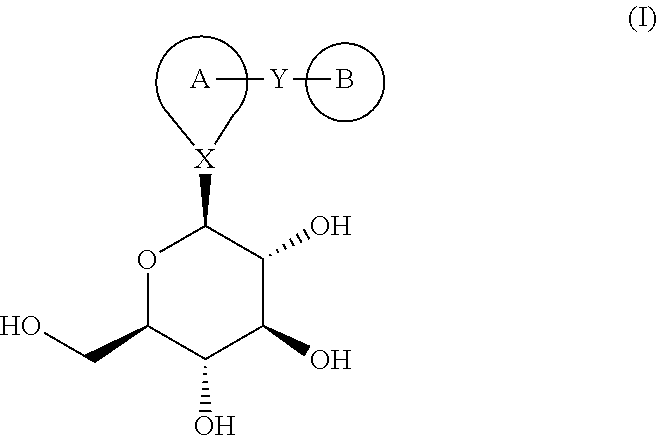
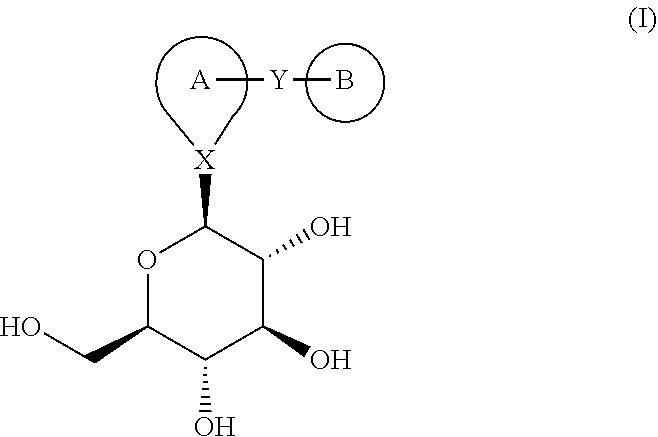
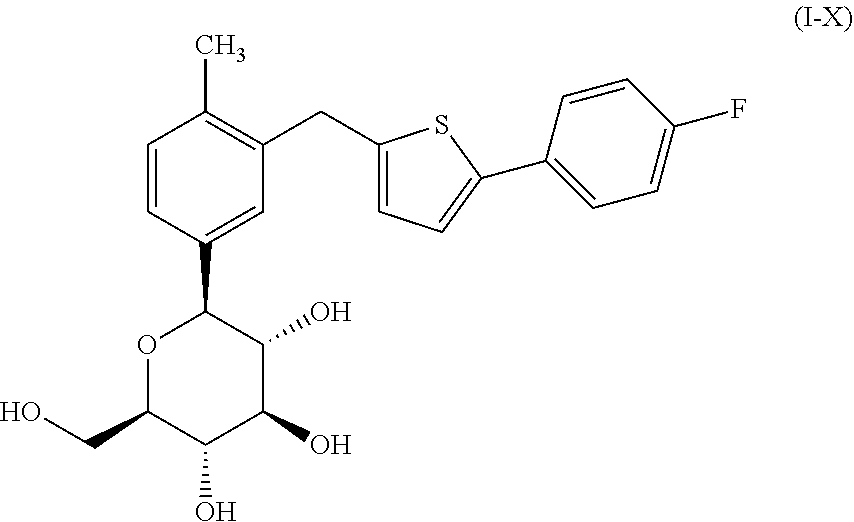
Combination therapy for the treatment of diabetes
a combination therapy and diabetes technology, applied in the field of cotherapy and methods for the treatment and prevention of glucose-related disorders, can solve problems such as glucose build-up in the blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Vivo Mouse Study
[0284]Male C57BL / 6 mice (total of 100 mice) were fed with a high fat diet from the age of 3 weeks to 6 weeks (total 3 weeks). After 3 weeks on a high fat diet, all the mice received a single-dose ip injection of Steptozotocin (STZ) (100 mg / kg, in 0.05 mol / L citric acid, pH4.5, 10 mg / ml). All the mice were then kept on a high fat diet for another 3 weeks. Those mice with fasted blood glucose levels >7 mM and <15 mM were selected for the study.
[0285]At the start of the pre-dose period, each mouse was assigned a predose number, which was indicated on its cage card. After assignment to dosage groups, each mouse was assigned a unique study identification number (which will be indicated on its cage card) and identified by permanent marker on its tail. Mice were housed 5 mice per cage in stainless steel cages. The study room was maintained on a 12-hour light / dark cycle (light / dark cycle may be interrupted for study-related activities), within a temperature range of 64° F...
example 2
In Vivo Mouse Study
[0293]Male ob / ob mice (8-week old, ˜50 g) were housed 2 mice per cage in a temperature-controlled room with 12-hour light / dark cycle. The mice were allowed ad libitum access to water and chow (commercially supplied diet). Mice were grouped into 6 test groups based on their body weight and fed blood glucose levels, as noted in Table 2, below.
TABLE 2Mouse Treatment groupsGroupTreatment1Vehicle (0.5% Methocel) 1 ml / 100 g, P.O.2Compound of formula (I-X) @ 1.0 mpk: 1 ml / 100 g, P.O.3Compound of formula (I-X) @ 10.0 mpk: 1 ml / 100 g, P.O.4Metformin HCl: 250 mpk: 1 ml / 100 g, P.O.5Compound of formula (I-X) @ 1 mpkMetformin HCl: 250 mpk, 1 ml / 100 g, P.O.6Compound of formula (I-X) @ 10 mpkMetformin HCl: 250 mpk, 1 ml / 100 g, P.O.
Study Design:
[0294]On the first morning, the mice were grouped as noted above and fed glucose. The mice were then dosed with vehicle or test compound(s) via gavage at 4:00 pm each day for 22 days q.d. The compound of formula (I-X) was dosed at 1 mg / kg ...
example 3
Pharmaceutical Composition
Combination of Metformin Hydrochloride and the Compound of Formula (I-X)
[0299]A pharmaceutical composition comprising metformin hydrochloride and the compound of formula (I-X) was prepared as follows, with Table 4, below listing the components in the formulation. Metformin HCl was purchased as commercially available Drug Substance (DS) from Solmag S. P. A Mulazzano (Via Della Vittoria 89, 26837 Cassino d'Alberi, Mulazzano, Italy).
TABLE 4Combination Tablet Formulationmg / Quanity / DescriptionFunctiontablet% w / wBatch (g)Intragranular AdditionsCompound of FormulaDrug200.014.69132.2(I-X)Substance-1Metformin HClDrug1000.073.46660.8Substance-2Microcrystalline CelluloseFiller59.24.3539.1Povidone (K29 / 32)1Binder54.504.0036.0Croscarmellose sodiumDisintegrant40.803.0027.3Water2NAN / AExtragranular AdditionsMagnesium Stearate, 2257Lubricant6.80.504.5Totals100.01361.3100.0899.61Added as 6% solids in solution2Not in final formulation
[0300]Metformin hydrochloride, the compoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com