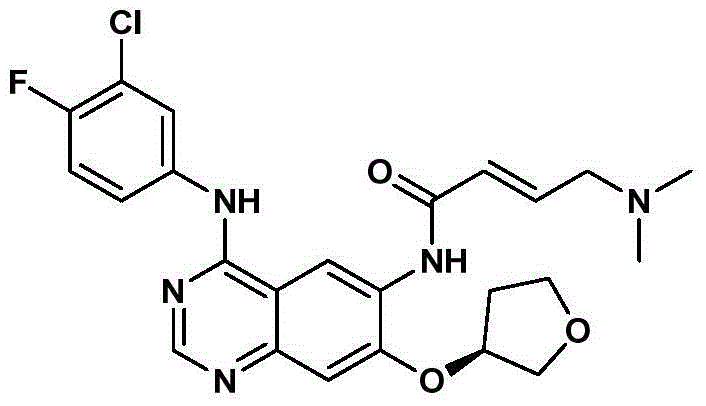
Synthetic method for afatinib intermediate
A compound, fluorophenyl technology, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of cumbersome processing, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
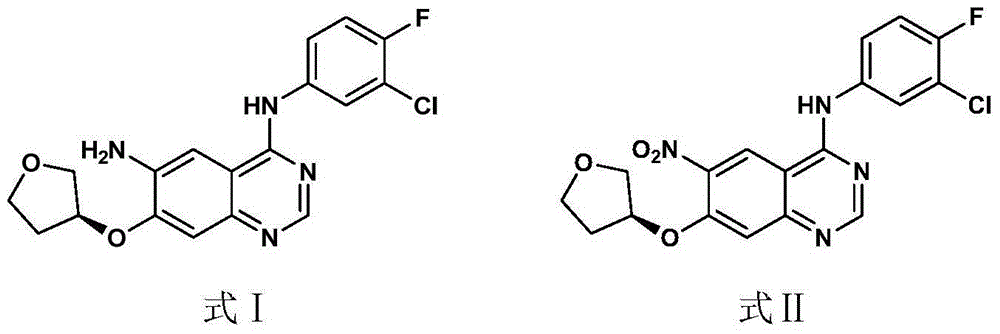
[0031] Synthesis of Example 1 (S)-4-(3-chloro-4-fluorophenyl)-7-[(tetrahydrofuran-3-yl)oxy]quinazoline-4,6-diamine
[0032]
[0033] Add (S)-N-(3-chloro-4-fluorophenyl)-6-nitro-7-[(tetrahydrofuran-3-yl)oxy]quinazolin-4-amine ( 50g, 0.124mol), add hydrosulfite (75g, 0.432mol), ethanol 250ml, water 100ml, heat up to 55°C for 1h, stop heating, cool down to room temperature, add 50% NaOH 60ml to adjust alkalinity under ice bath cooling, solid Precipitated, filtered, and dried to obtain 41.2 g of a light green solid, with a yield of 89% and a liquid phase purity of 98%.
[0034] 1 H-NMR (300Hz, DMSO-d6) δ: 9.39 (s, 1H, NH), 8.38 (s, 1H, ArH), 8.18-8.20 (m, 1H, ArH), 7.80-7.82 (m, 1H, ArH ), 7.36~7.41(m, 2H, ArH), 7.06(s, 1H, ArH), 5.38(s, 2H, NH 2 ), 5.23(s, 1H, 1 / 2CH 2 ), 3.89~4.03 (m, 3H, CH 2 ,1 / 2CH 2 ),3.76~3.83(m, 1H, 1 / 2CH 2 ),2.29~2.38(m,1H,1 / 2CH 2 ), 2.10~2.15(m, 1H, 1 / 2CH 2 ).
Embodiment 2
[0036] Add (S)-N-(3-chloro-4-fluorophenyl)-6-nitro-7-[(tetrahydrofuran-3-yl)oxy]quinazolin-4-amine ( 100.0g, 0.247mol), ethanol (1600ml), water (400ml) and glacial acetic acid (140ml), add reduced iron powder (55.0g, 0.99mol) after heating to reflux, and continue to reflux for 2 hours, and TLC detects that the reaction of the raw materials is complete ;Stop heating, lower the temperature to about 50°C (external temperature), filter the reaction solution through diatomaceous earth, and then filter the filtrate once through silica gel (100-200 mesh); spin the filtrate to dryness, and add 1L of dichloro Methane, after stirring evenly, suction filtration; spin dry the filtrate, add about 400ml ether to the residue, stir for 30min, suction filtration; filter cake is air-dried at 50°C, the yield is 70%, and the liquid phase purity is 97%.
Embodiment 3
[0038] Add (S)-N-(3-chloro-4-fluorophenyl)-6-nitro-7-[(tetrahydrofuran-3-yl)oxy]quinazolin-4-amine ( 5g, 0.012mol), ammonium chloride (0.89g, 0.0168mol), DMF50ml, then add Raney-Ni0.5g, hydrogenation reaction at room temperature for 2h, after the reaction is completed, diatomaceous earth filter, the reaction solution is poured into water, solid precipitates, filter , a light green solid was obtained with a liquid phase purity of 31.8%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap