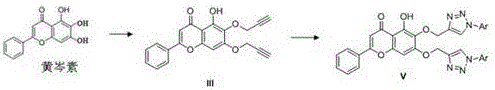
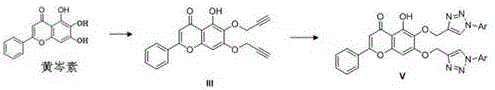
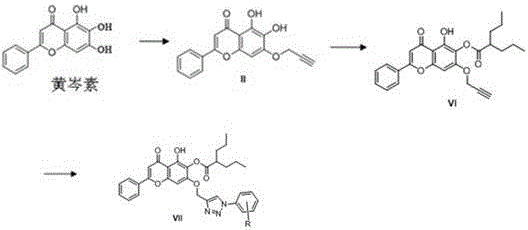
Baicalein derivative and preparation method thereof
A technology for baicalein and derivatives, which is applied in the field of medicine, can solve the problems of poor water solubility, low bioavailability, limited application and the like of baicalein, and achieves the effects of low cost, simple reaction operation and easy handling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Preparation of (Compound IVb)
[0043] The preparation of compound IVb is similar to the preparation of compound IVa, substituting 1-azido-4-methylbenzene with 1-azido-2-methoxybenzene; 1 H NMR (400 MHz, DMSO- d 6 ) δ 12.57 (s, 1H), 7.93 – 7.89 (m, 2H), 7.59 – 7.49 (m, 3H), 7.46 – 7.40 (m, 1H), 7.40 – 7.31 (m, 3H), 6.93 (s, 1H) ), 6.69 (s, 1H), 5.61 – 5.41 (m, 3H), 2.22 (s, 3H); 13 C NMR (101 MHz, DMSO- d 6 ) δ 182.38,163.32,153.21,149.61,146.46,142.21,136.18,133.04,132.02,131.43,130.86,130.38,129.92,129.17,127.06,126.65,126.36,126.03,105.60,104.76,92.69,62.31,17.44; HRMS ( m / z): calcd for C 25 H 20 N 3 O 5 [M+H] + : 442.1397, found: 442.1397.
[0044] Example 5: 5,6-Dihydroxy-2-phenyl-7-((1-( m -tolyl)-1 H -1,2,3-triazol-4-yl)methoxy)-4 H -chromen-4-one (FZU-0016-021)
[0045] Preparation of (Compound IVc)
[0046] Compound IVc was prepared analogously to compound IVa, substituting 1-azido-4-methylbenzene for 1-azido-3-methoxybenzene. 1 H NMR (400...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap