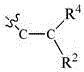
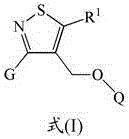
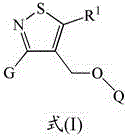
Isothiazole derivatives as gpr120 agonists for the treatment of type ii diabetes
A compound and substituent technology, applied in the field of novel isothiazole and thiophene derivatives, can solve problems such as increasing the potential of CLP-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0355] 3-(4-[[3-(4-chlorophenyl)-5-(trifluoromethyl)-1,2-thiazol-4-yl]methoxy]-2,3-difluorophenyl) Propionic acid, compound 106
[0356] Step 1: 5-(4-Chlorophenyl)-2H-1,3,4-oxthiazol-2-one
[0357]
[0358]To a 100 mL round bottom flask was added 4-chlorobenzamide (3.0 g, 19.28 mmol, 1.00 equiv), chloro(chlorosulfonyl)methanone (5.03 g, 38.40 mmol, 1.99 equiv), toluene (30 mL). The resulting solution was stirred overnight in a 100°C oil bath. The progress of the reaction was monitored by GCMS / TLC / LCMS (ethyl acetate / petroleum ether=1:20). The resulting mixture was concentrated in vacuo. The residue was applied to a silica gel column and eluted with petroleum ether. This gave 3.8 g (92%) of 5-(4-chlorophenyl)-2H-1,3,4-oxthiazol-2-one as a white solid.
[0359] Step 2: Ethyl 3-(4-chlorophenyl)-5-(trifluoromethyl)-1,2-thiazole-4-carboxylate
[0360]
[0361] Add 5-(4-chlorophenyl)-2H-1,3,4-oxthiazol-2-one (2.2 g, 10.30 mmol, 1.00 equiv), 4,4,4-trifluorobutane t...
Embodiment 2
[0375] 3-(4-[[3-(4-ethylphenyl)-5-(trifluoromethyl)-1,2-thiazol-4-yl]methoxy]-3,5-difluorobenzene base) propionic acid, compound 70
[0376] Step 1: 5-(4-Ethylphenyl)-1,3,4-oxthiazol-2-one
[0377]
[0378] The title compound was prepared according to the procedure described in Example 1, Step 1, using 4-ethylbenzamide as starting material to afford the desired product as a yellow solid.
[0379] Step 2: Ethyl 3-(4-ethylphenyl)-5-(trifluoromethyl)-1,2-thiazole-4-carboxylate
[0380]
[0381] To a 50 mL sealed test tube was added ethyl 4,4,4-trifluorobut-2-ynoate (1.25 g, 7.53 mmol, 1.56 equiv), 5-(4-ethylphenyl)-2H-1,3, 4-oxthiazol-2-one (1.0 g, 4.83 mmol, 1.00 equiv), 1,3-dichlorobenzene (8 mL). The resulting solution was stirred at 150 °C for 16 h. The progress of the reaction was monitored by LCMS. The resulting mixture was concentrated in vacuo. The residue was applied to a silica gel column and eluted with petroleum ether (100). This gave 1.068 g (67%)...
Embodiment 3
[0395] 3-(4-[[3-(4-chlorophenyl)-5-(trifluoromethyl)-1,2-thiazol-4-yl]methoxy]-3,5-difluorophenyl) Propionic acid, compound 79
[0396]
[0397] The title compound was prepared according to the following procedure described in Example 2: Starting from (3-(4-ethylphenyl)-5-(trifluoromethyl)isothiazol-4-yl)methyl methanesulfonate, Following steps 5 and 6, using tert-butyl 3-(3,5-difluoro-4-hydroxyphenyl)propionate as a coupling agent, the desired product was obtained as an off-white solid. 1 H NMR (300MHz, CD 3 OD) δ7.77(d, J=8.7Hz, 2H), 7.51(d, J=8.4Hz, 2H), 6.86(d, J=9.6Hz, 2H), 5.20(s, 2H), 2.87(t , J=7.5Hz, 2H), 2.59(t, J=7.5Hz, 2H). Mass Spectrum (ESI, m / z): for C 20 h 13 CIF 5 NO 3 S, calculated value is 478.0 (M+H), found value is 478.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap