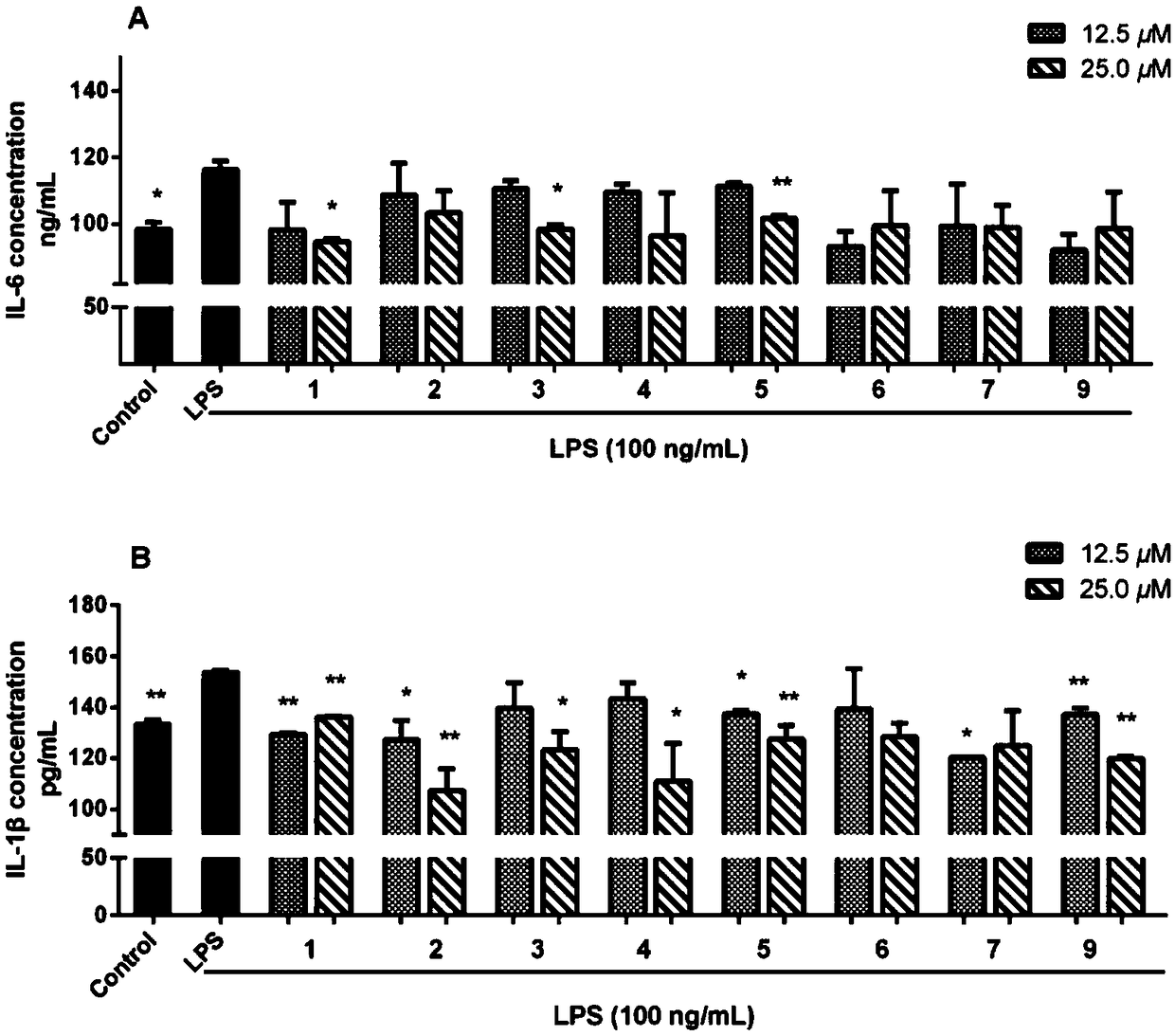
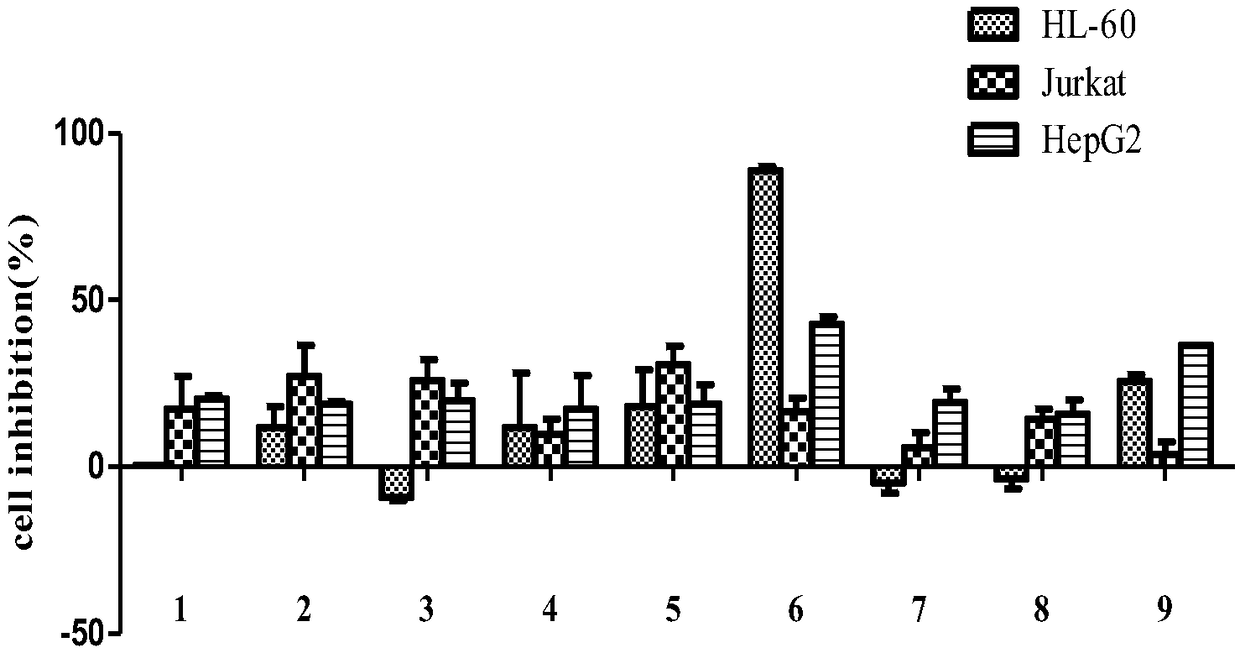
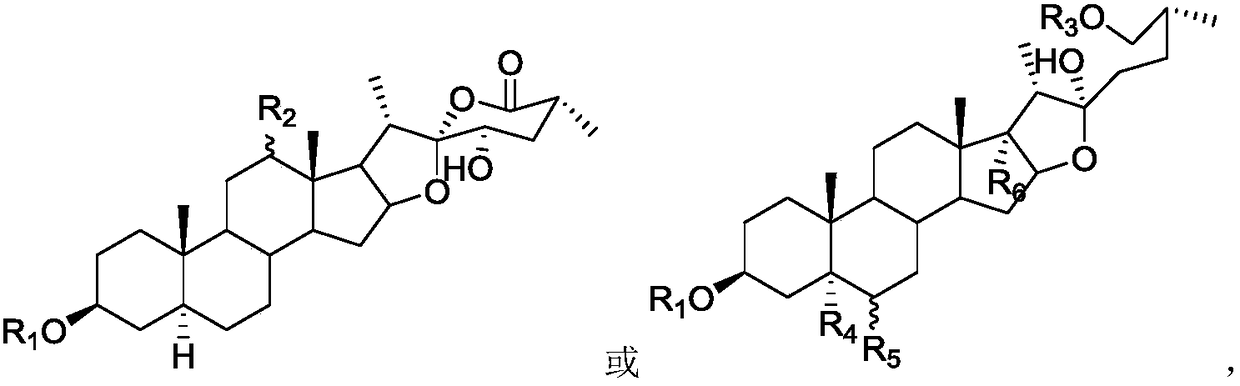
Novel steroidal saponins and their applications
A technology of steroidal saponins and compounds, applied in the field of medicine, can solve problems such as insufficient research on the chemical constituents of Solanum nigrum, chemical structure and anti-inflammatory and anti-tumor activities have not been reported, and achieve good in vitro anti-inflammatory activity, chemically defined effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The extraction of embodiment 1 steroidal saponins
[0032] Take 6 kg of dried Solanum solanum fruit, extract twice with cyclohexane under reflux, and discard the cyclohexane extract. The medicinal material after degreasing treatment is heated and refluxed with 10 times the amount of 70% methanol and extracted three times for 2 hours each time, and the combined extracts are concentrated under reduced pressure to obtain the extract. Suspend the extract in water, pass through a D101 macroporous resin column (1300×100mm), and elute with water, 10% methanol, 30% methanol, 50% methanol, 70% methanol and 100% methanol, among which 50% methanol Compound 1-9 was isolated by repeated silica gel column chromatography, reverse-phase MPLC column chromatography, Sephadex LH-20 column chromatography and semi-preparative reverse-phase HPLC column chromatography.
Embodiment 2
[0033] Identification of Example 2 Compound 1
[0034] By means of physical and chemical constants and spectroscopy (HRESIMS, 1 H-NMR, 13 C-NMR, HSQC, HMBC and 1 H- 1 H COZY spectrum) to identify compound 1, the results are as follows:
[0035] Compound 1 is a white amorphous powder, The reaction to Anisaldehyde (A reagent) showed yellow color, but to Ehrlish (E reagent) showed no color, and acid hydrolysis detected D-glucose and D-galactose, suggesting that the compound was a spirosteroid saponin compound. HRESIMS (positive) gives the quasi-molecular ion peak [M+NH 4 ] + m / z 1128.5492 (calcd.for C 51 h 86 NO 26 1128.5438), suggesting that its molecular weight is 1110, combined with 1 H-NMR and 13 C-NMR (DEPT) can determine its molecular formula as C 51 h 82 o 26 .
[0036] exist 1 In the H-NMR spectrum, the high-field region gives four characteristic methyl signals on steroidal saponins, two of which are single peaks, and the other two are double peaks, resp...
Embodiment 3
[0042] Identification of Example 3 Compound 2
[0043] By means of physical and chemical constants and spectroscopy (HRESIMS, 1 H-NMR, 13 C-NMR, HSQC, HMBC and 1 H- 1 H COZY spectrum) to identify compound 2, the results are as follows:
[0044] Compound 2 is a white amorphous powder, The reaction to Anisaldehyde (A reagent) showed yellow color, but to Ehrlish (E reagent) showed no color, and acid hydrolysis detected D-glucose and D-galactose, suggesting that the compound was a spirosteroid saponin compound. HRESIMS (positive) gives the quasi-molecular ion peak [M+NH 4 ] + m / z 1128.5497 (calcd.forC 51 h 86 NO 26 1128.5438), suggesting that its molecular weight is 1110, combined with 1 H-NMR and 13 C-NMR (DEPT) can determine its molecular formula as C 51 h 82 o 26 .
[0045] exist 1 In the H-NMR spectrum, the high-field region gives four characteristic methyl signals on steroidal saponins, two of which are single peaks and the other two are double peaks, respec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com