Beta-carboline compound and synthesizing method and application thereof
A synthetic method and compound technology, applied in the field of medicine, can solve the problems of limited production cost, insufficient research on β-carboline alkaloids, and low content, and achieve good medicinal value and significant anti-renal fibrosis activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 structure is as the preparation of the compound shown in formula I
[0031] 1) Dissolve 200 mg (9.3 mmol) of NaH in 1 mL of N, N-dimethylformamide, stir for 10 min, and gradually add 275 mg (1 mmol) of 1-pyridine-6-methoxy-β-carboline, Stir at room temperature for 10 minutes, then add 391.5 mg (1.5 mmol) of 2,3,4,5-tetrafluorobenzyl bromide dropwise, and continue the reaction at room temperature for about 20 minutes; after the reaction, the product obtained is adjusted to neutrality with 300 ml of ammonia water , and extracted with ethyl acetate, the organic phase was collected, and the solvent was removed under reduced pressure to obtain a yellow oily crude product;
[0032] 2) The crude product was subjected to silica gel column chromatography using a solvent composed of petroleum ether:ethyl acetate=1000:100 (volume ratio) as an eluent to obtain a pale yellow flocculent product (347mg, yield 79.4%, purity 99.94%) .
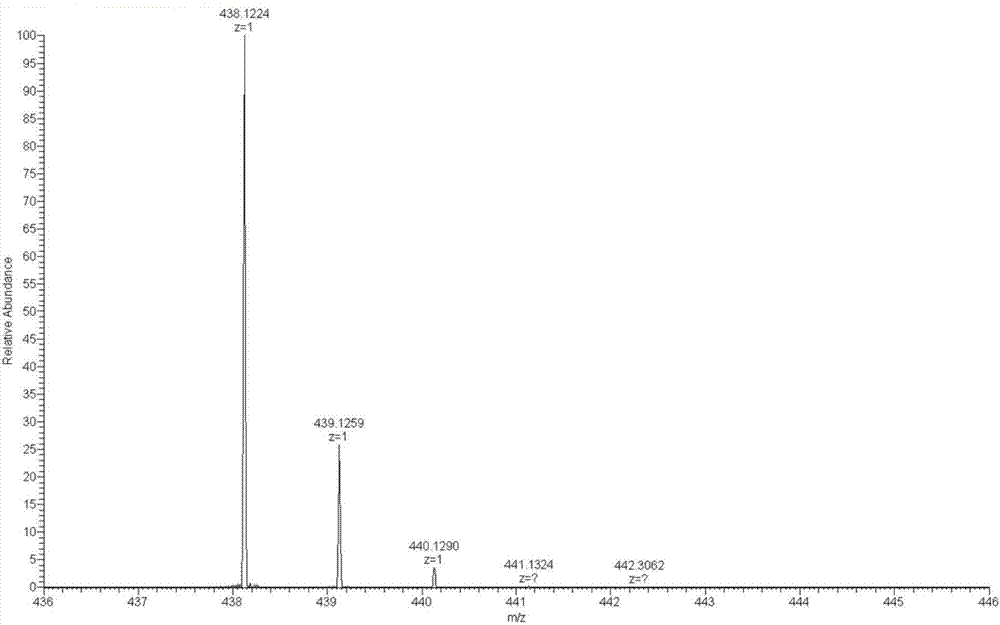
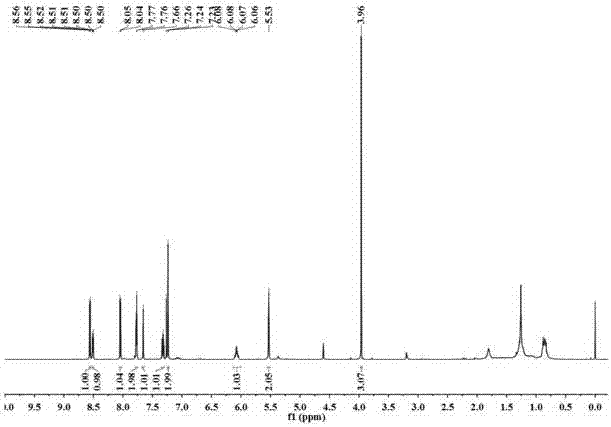
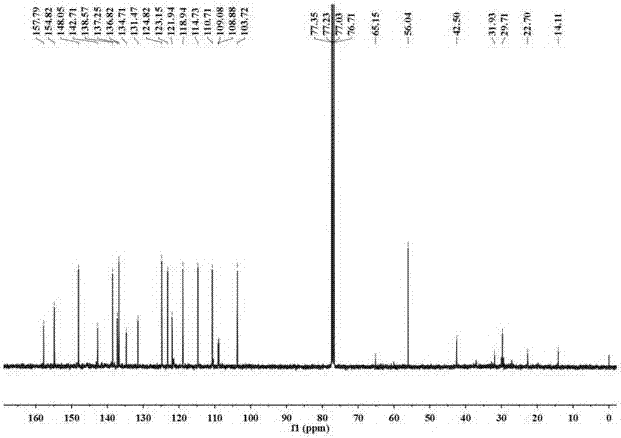
[0033] The resulting pale yellow fl...
Embodiment 2
[0039] Embodiment 2 structure is as the preparation of the compound shown in formula I
[0040] Dissolve 400mg (16.6mmol) of NaH in 1mL N, N-dimethylformamide, stir for 10min, and gradually add 550mg (2mmol) of 1-pyridine-6-methoxy-β-carboline, stir at room temperature 10min, then add 391.5mg (1.5mmol) of 2,3,4,5-tetrafluorobenzyl bromide dropwise, and continue the reaction at room temperature for about 20min; Extracted with ethyl acetate, collected the organic phase, and removed the solvent under reduced pressure to obtain the crude product of yellow oily liquid;
[0041] The crude product was subjected to silica gel column chromatography using petroleum ether:ethyl acetate=1000:100 (volume ratio) as the eluent to obtain a light yellow flocculent product (211 mg, yield 48.3%, purity 99.91%).
[0042]The obtained pale yellow flocculent product was characterized by NMR, and was determined to be 1-pyridine-6-methoxy-9-(2,3,4,5-tetrafluorobenzyl)β-carboline described in the pres...
Embodiment 3
[0043] Embodiment 3 structure is as the preparation of the compound shown in formula I
[0044] 1) Dissolve 200 mg (9.33 mmol) of NaH in 5 mL of N, N-dimethylformamide, stir for 10 min, and gradually add 275 mg (1 mmol) of 1-pyridine-6-methoxy-β-carboline, Stir at room temperature for 10 min, then add 524 mg (2 mmol) of 2,3,4,5-tetrafluorobenzyl bromide dropwise, and continue the reaction at room temperature for about 20 min; Extracted with ethyl acetate, collected the organic phase, and removed the solvent under reduced pressure to obtain the crude product of yellow oily liquid;
[0045] 2) The crude product was subjected to silica gel column chromatography using a solvent composed of petroleum ether:ethyl acetate=1000:50 (volume ratio) as an eluent to obtain a pale yellow flocculent product (162mg, yield 37.1%, purity 99.90%) .
[0046] The obtained pale yellow flocculent product was characterized by NMR, and was determined to be 1-pyridine-6-methoxy-9-(2,3,4,5-tetrafluoro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com