Ruthenium and rhodium metal complexes taking lysicamine as ligands as well as synthetic method and application of ruthenium and rhodium metal complex
A technology of Guanyin Lianming base and metal complexes, which is applied in the field of medicine and can solve the problems that the research on pharmacological activity is still blank
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Synthesize Ru with solution method Ⅱ -LY complex
[0037] Take 1mmol RuCl 2 (DMSO) 4 Dissolve in 10ml of chloroform, dissolve 1mmol of LY in 15mL of methanol, mix and react the two solutions at 65°C for 12h, and obtain a dark green solution after the reaction; part of the solvent is evaporated from the solution to make the product reach supersaturation and precipitate, A dark green solid was isolated, washed and dried to give the product as a dark green solid (yield 93%).
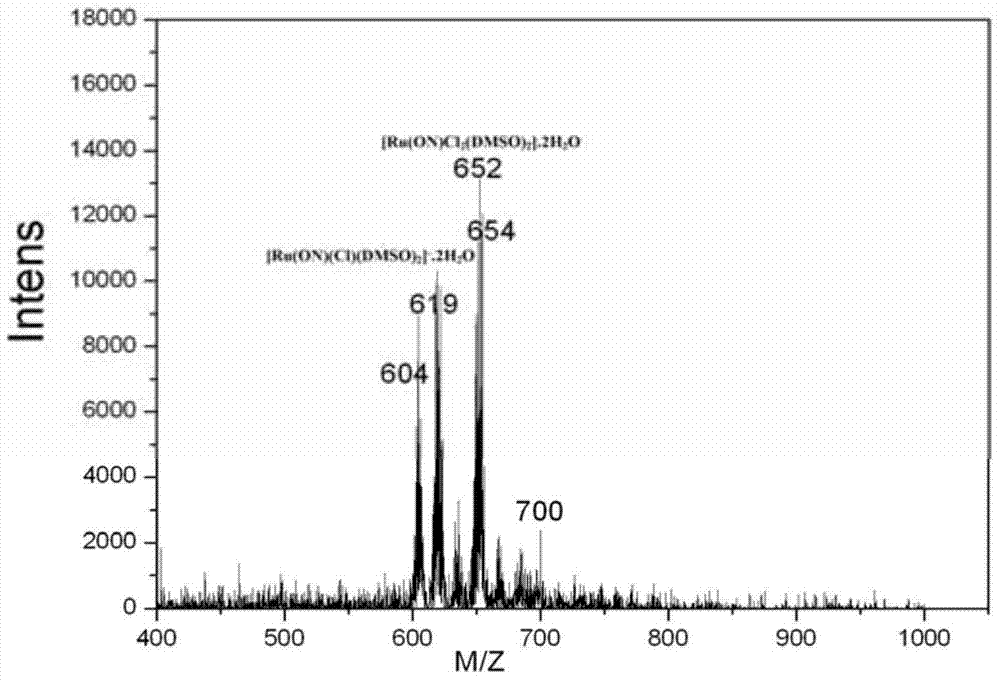
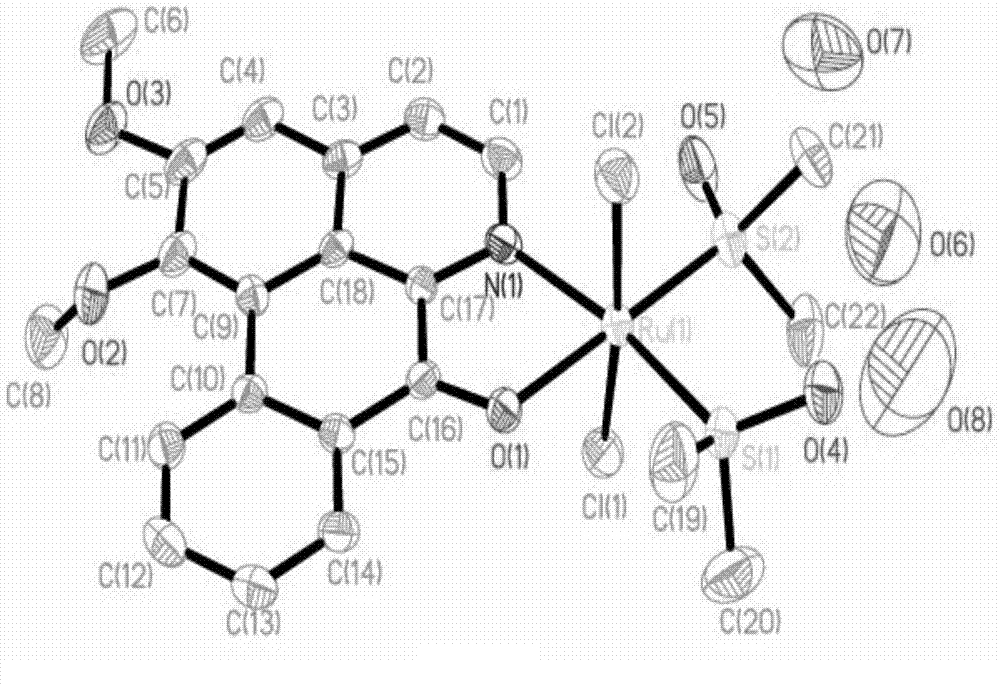
[0038] Infrared spectroscopy, elemental analysis, electrospray mass spectrometry, and single crystal diffraction analysis were carried out on the obtained dark green solid. The specific spectral characteristics are as follows:
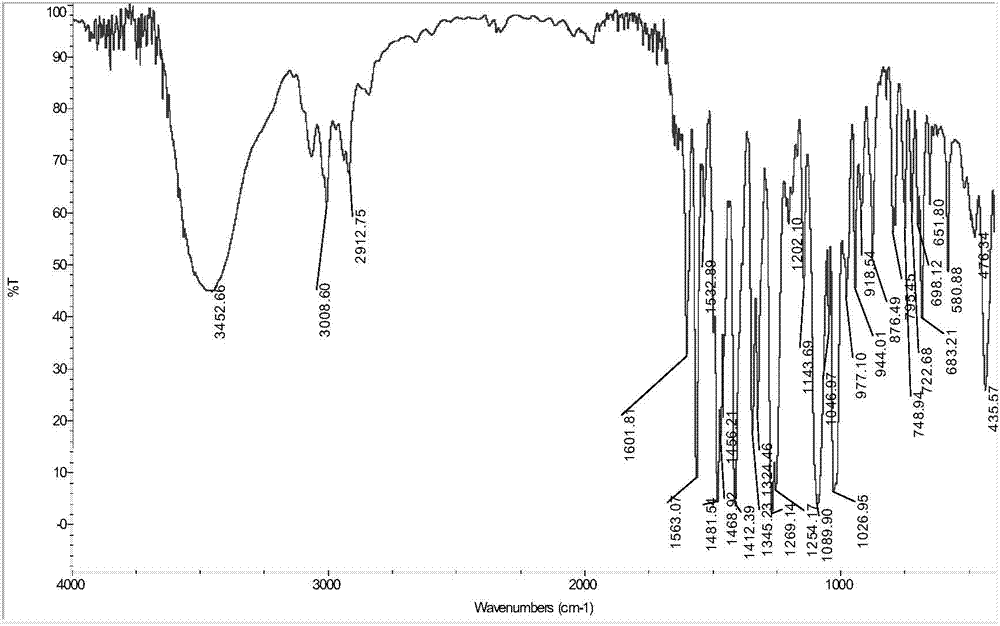
[0039] (1) Infrared spectrum, its spectrogram is as follows figure 1 As shown, IR(KBr):(Ar-H)3008(m),(C=O)1601(m),(C=C)1563 / 1481 / 1468(s),(C-O)14121269(vs), (C-N)1026(s)cm -1 .
[0040] (2) elemental analysis, Anal.Calc.(for C 22 h 31 Cl 2 NO 8 RuS 2 ...
Embodiment 2
[0044] Embodiment 2: Synthesize Rh with solution method Ⅲ -LY complexes:
[0045] Take 0.1mmol RhCl 3 ·3H 2 O was dissolved in 10ml of water, 1mmol of LY was dissolved in 15mL of ethanol, and the reaction was stopped at 80°C for 12h under reflux, cooled to room temperature, and left standing overnight; filtered, the filtrate slowly evaporated at room temperature, two weeks later Red square-shaped crystals were precipitated, and the crystals were separated, washed and dried to obtain dark red square-shaped crystals (93% yield).
[0046] Infrared spectroscopy, elemental analysis, electrospray mass spectrometry, and single crystal diffraction analysis were carried out on the obtained deep red. The specific spectral characteristics are as follows:
[0047] (1) Infrared spectrum, its spectrogram is as follows Figure 4 as shown,
[0048] IR(KBr)::(Ar-H)3066(m),(C=O)1602(m),(C=C)1557 / 1496 / 1429(s),(C-O)127812143(vs),(C-N )1077(s)cm -1
[0049] (2) elemental analysis, Anal.Cal...
Embodiment 3
[0055] Embodiment 3: solvothermal synthesis of Ru Ⅱ -LY metal complexes
[0056] In a thick-walled glass tube with an open end, add 0.1 mmol of the intermediate product RuCl 2 (DMSO) 4 and 0.1mmol of LY, then add 2mL of acetone and 1mL of dichloromethane mixed solvent, under vacuum conditions, seal the other open end, and then react at 80°C for 48h, remove the solvent after the reaction, take out the deep The green solid was washed with ethanol to remove unreacted substances and impurities, and dried in vacuum at 40°C for 4 hours to obtain a dark green solid. This product was determined to be the complex [C 22 h 31 Cl 2 NO 8 RuS 2 ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com