Beta-carboline compound as well as synthesis method and application thereof
A synthesis method and compound technology, applied in the field of medicine, can solve the problems of limited preparation cost, insufficient expansion research of β-carboline alkaloids, in-depth research, etc., and achieve good medicinal value and significant anti-renal fibrosis activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The structure of Example 1 is the preparation of the compound shown in formula I
[0031] 1) Dissolve 200mg (8.3mmol) of NaH in 1mL N,N-dimethylformamide, stir for 10min, and gradually add 275mg (1mmol) of 1-pyridine-6-methoxy-β-carboline, Stir at room temperature for 10 minutes, then add 445 mg (1.5 mmol) of 3-iodobenzyl bromide dropwise, and continue the reaction at room temperature for about 20 minutes; after the reaction, the product obtained is adjusted to neutral with 300 ml of ammonia, and then extracted with ethyl acetate, and collected In the organic phase, the solvent was removed under reduced pressure to obtain a crude product as a yellow powder;
[0032] 2) The crude product uses a solvent composed of petroleum ether: ethyl acetate=1000:100 (volume ratio) as the eluent, and silica gel column chromatography is used to obtain a pale yellow flocculent product (400mg, yield 81.6%, purity 99.92%) .
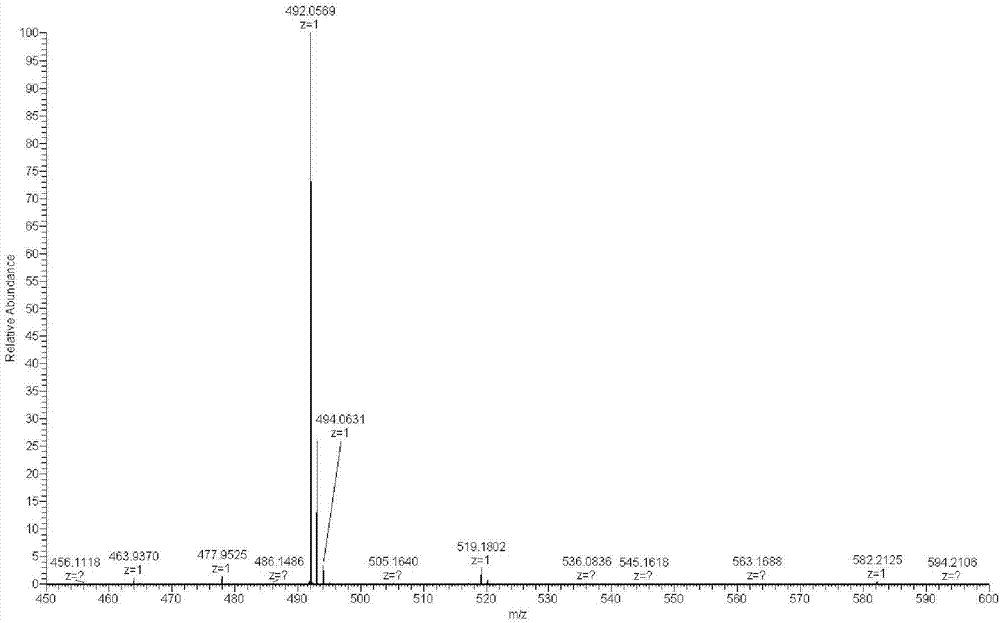
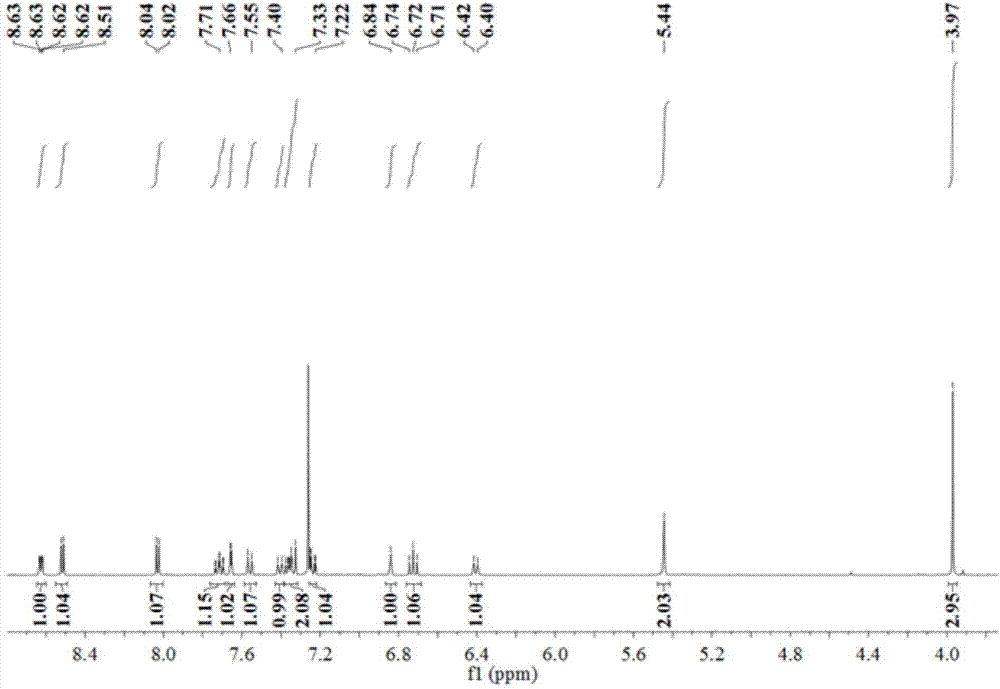
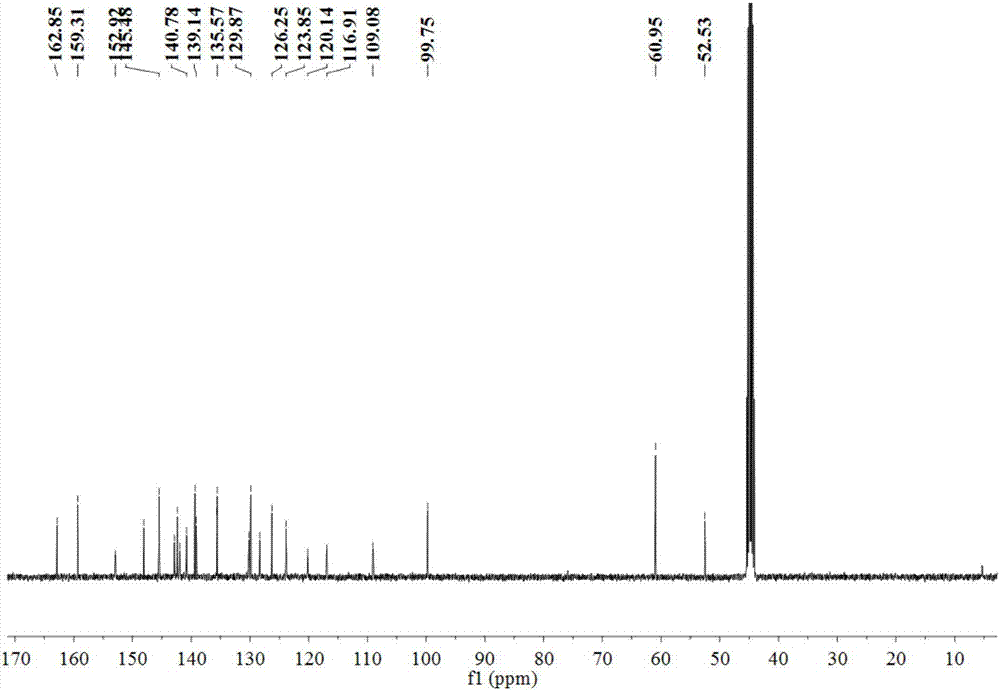
[0033] The resulting pale yellow flocculent product was characterize...
Embodiment 2
[0039] The structure of Example 2 is the preparation of the compound shown in formula I
[0040] 1) Dissolve 400mg (16.6mmol) of NaH in 1mL of N,N-dimethylformamide, stir for 10min, and gradually add 550mg (2mmol) of 1-pyridine-6-methoxy-β-carboline, Stir at room temperature for 10 minutes, then add 445 mg (1.5 mmol) of 3-iodobenzyl bromide dropwise, and continue the reaction at room temperature for about 20 minutes; after the reaction, the product obtained is adjusted to neutrality with 400 ml of ammonia, extracted with ethyl acetate, and the organic phase is collected , The solvent was removed under reduced pressure to obtain the crude product as a yellow oily liquid;
[0041] 2) The crude product uses a solvent composed of petroleum ether: ethyl acetate=1000:80 (volume ratio) as the eluent, and silica gel column chromatography is used to obtain a pale yellow flocculent product (381 mg, yield 51.6%, purity 99.90%) .
[0042] The obtained light yellow flocculent product was charac...
Embodiment 3
[0043] The structure of Example 3 is the preparation of the compound shown in formula I
[0044] 1) Dissolve 200mg (8.33mmol) of NaH in 5mL N,N-dimethylformamide, stir for 10min, and gradually add 275mg (1mmol) of 1-pyridine-6-methoxy-β-carboline, Stir at room temperature for 10 minutes, and then add 593mg (2mmol) of 3-iodobenzyl bromide dropwise, and continue the reaction at room temperature for about 20 minutes; after the reaction, the resulting reactant is adjusted to neutral with 400ml ammonia water, extracted with ethyl acetate, and the organic phase is collected , The solvent was removed under reduced pressure to obtain the crude product as a yellow oily liquid;
[0045] 2) The crude product uses the solvent composed of petroleum ether: ethyl acetate=1000:50 (volume ratio) as the eluent, and silica gel column chromatography is used to obtain a light yellow flocculent product (440mg, yield 89.8%, purity 99.95%) .
[0046] The obtained light yellow flocculent product was charac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap