Tylvalosin tartrate soluble powder and preparation method thereof
A technology of tyvanectin and tartaric acid, which is applied in powder delivery, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems that soluble powder cannot be directly applied, and achieve good inclusion effect, strong operability, and high solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, preparation tyvalactin tartrate soluble powder
[0029] The preparation method of the tyvalactin tartrate soluble powder of the present embodiment comprises:
[0030] The first step is to mix hydroxypropyl-β-cyclodextrin with water and stir until dissolved to obtain the first liquid; the weight ratio of hydroxypropyl-β-cyclodextrin to water is (0.8-1.2):(16 -twenty four);
[0031] Wherein, the process of preparing the first liquid is carried out in a water bath with a temperature lower than or equal to 15°C. The weight ratio of hydroxypropyl-β-cyclodextrin to water is preferably 1:(20-24).
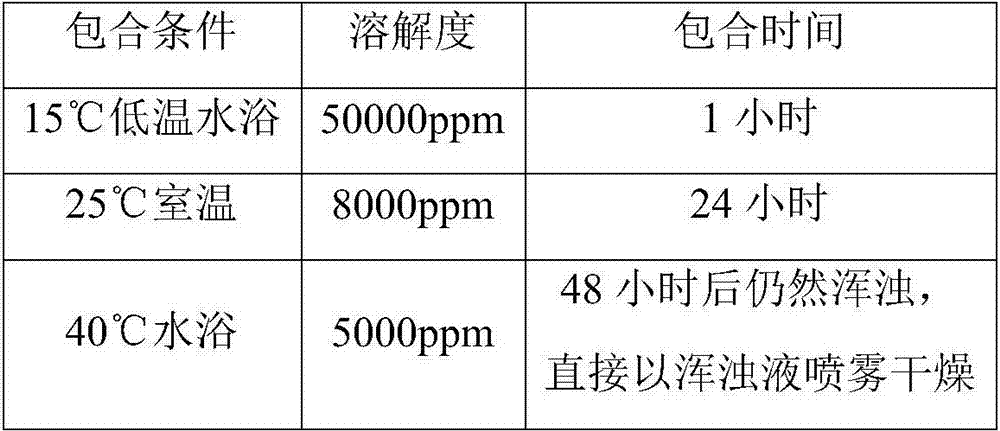
[0032] In the second step, in a water bath with a temperature less than or equal to 15°C, mix the first liquid, the raw material of tyvanectin tartrate, and the pH regulator tartaric acid, and stir for at least 0.75 hours until a clear liquid is obtained; The weight ratio of medicine and pH regulator tartaric acid is 1:(0.015-0.025); The weight ratio of hydroxypropy...
Embodiment 2
[0038] Embodiment 2, solubility detection
[0039] The finished product of the test case of Example 1, the bulk drug of tyvalomectin tartrate, and the soluble powder of tyvalomectin tartrate in the market were used as samples. At 25°C, the solubility of the above samples in water was tested.
[0040] The results are shown in the table below:
[0041]
[0042] It can be seen that the solubility of the finished product of the test case of Example 1 is much higher than that of the tyvalomectin tartrate bulk drug and the commercially available tyvalomectin tartrate soluble powder.
Embodiment 3
[0043] Embodiment 3, stability monitoring
[0044] The finished product of the test case of Example 1, the bulk drug of tyvalomectin tartrate, and the soluble powder of tyvalomectin tartrate in the market were used as samples.
[0045] Each sample was placed under the conditions of 40°C and RH75% for 6 months, and its A component was monitored (A component not lower than 80% is qualified). Note: According to the first volume of the national standard for veterinary drugs "Tyvalamicin Tartrate Premix", and the imported veterinary drug standard "Tyvalamicin Tartrate Soluble Powder" approved and issued by the Ministry of Agriculture Announcement No. 1680, when investigating stability, the The main monitoring index is the content of component A (ie tylvanicin A), and no less than 80% of component A is qualified.
[0046] The results are shown in the table below:
[0047]
[0048] It can be seen that the stability of the finished product of the test case of Example 1 is ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com