Boric acid and borate compounds and application thereof
A compound and boric acid technology, applied in the direction of active ingredients of boron compounds, compounds containing elements of group 3/13 of the periodic table, drug combinations, etc., can solve problems such as no compound activity reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0190] Example 1 Preparation of intermediate S3-1 of the present invention and target compound I-1-1
[0191] The synthetic route is as follows:
[0192]
[0193] CN200780100142 discloses a method for synthesizing a compound similar to the present invention; it uses TBTU and the like as a condensing agent to react the condensation intermediate S1 of substituted benzoic acid and glycine with amino borate S2, but the inventors found , The compound disclosed in the present invention is prepared using the above-mentioned patent disclosure route, and the product obtained is mainly the by-product S4-1 of deboronic ester. The specific operation is as follows:
[0194] The starting material (S-1-1) 0.205g (0.70mmol) in 10mL DMF solution, add 0.248g (0.74mmol) and 0.267gS2 (0.70mmol, 1eq) of condensing agent TBTU, reduce the temperature to about 0 degrees, drop DIEA 0.367 mL (2.1 mmol). After the reaction, the organic layer was added with 100 mL of water, extracted with dichloromethane, drie...
Example Embodiment
[0208] Example 2 Preparation of compound I-1-2 of the present invention
[0209]
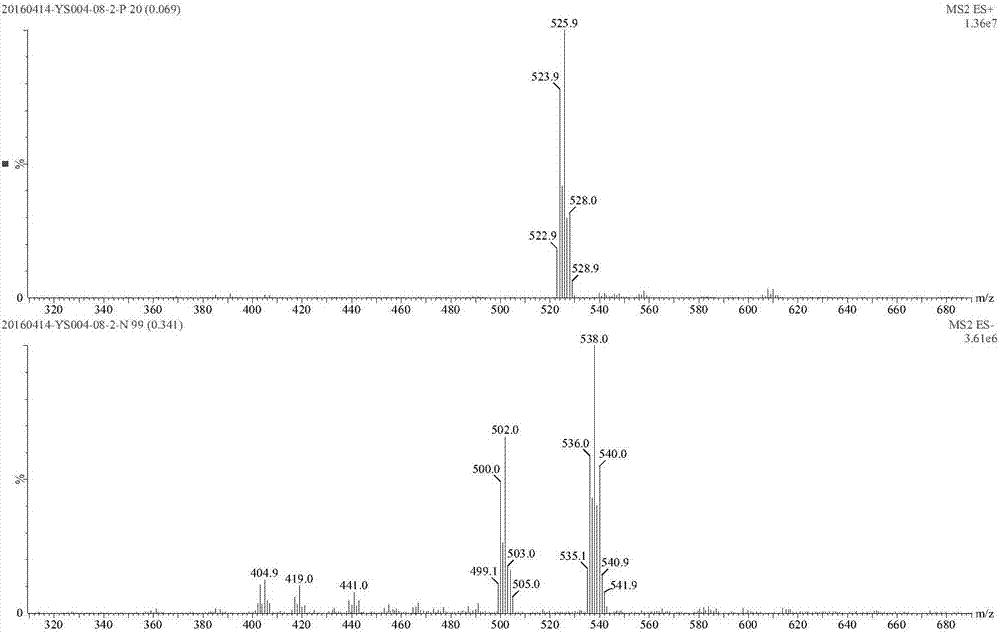
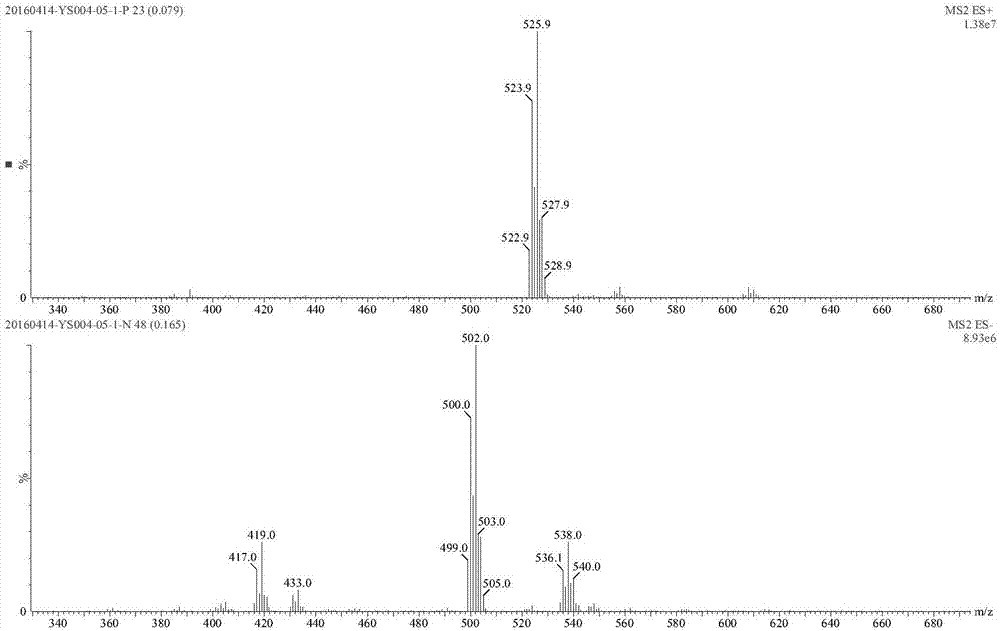
[0210] The boric acid starting material (I-1-1) 5g (12.3mmol), dipropanolamine (S-3-2, molecular weight 133.19) 1.95g (14.6mmol), 20mL ethyl acetate, stirred overnight at room temperature, precipitation A white solid was filtered to obtain 4.8 g of compound I-1-2 with a yield of 78%, namely 2-chloro-5-bromo-N-[(R)-1-[1,3,7,2]-dioxazepine Hetero-2-boryl-3-methyl-butanocarboxamido]-methyl]-benzamide.
[0211] 1 H NMR(300MHz,DMSO-d6)δ(ppm)8.95(brs,1H),7.63-7.68(m,2H),7.48-7.50(m,1H), 6.63(d,1H,J=8.61Hz), 4.81 (m, 1H), 3.87-3.92 (m, 1H), 3.75 (m, 1H), 3.65 (m, 4H), 3.20-3.34 (m, 3H), 2.66 (m, 2H), 1.89-1.99 ( m,1H),1.63-1.66(m,1H),1.49(m,2H),1.29-1.34(m,1H),1.15-1.23(m,1H),0.94-0.98(m,1H),0.83( d, 6H).
[0212] ESI m / z: 500.0[M-H] - .
[0213] Compound I-1-2 was obtained according to the above preparation process, and it was detected at a temperature of 20~25℃. The X-ray powder diffraction pattern of th...
Example Embodiment
[0215] Example 3 Preparation of compound I-1-3 of the present invention
[0216]
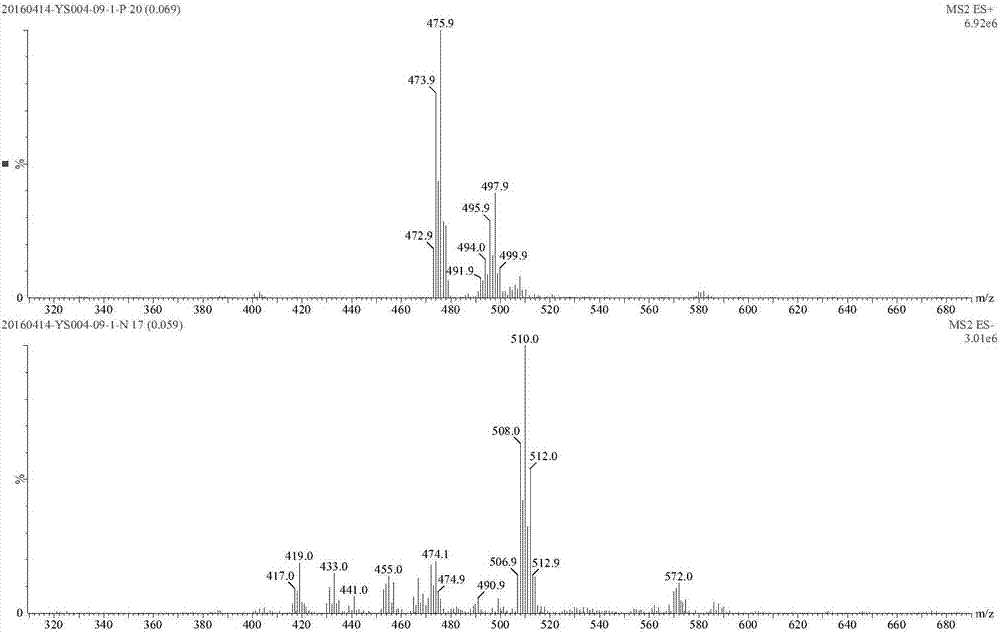
[0217] Dissolve 2.25 g (5.55 mmol) of boric acid raw material (I-1-1) in 45 ml of ethyl acetate, stir at room temperature for 5 min, and add 0.61 g (5.82 mmol) of diethanolamine (S-3-3) dropwise. A white solid precipitated in the reaction liquid. After the dropwise addition was completed, stirring was continued for 2 hours, and 2.4 g of compound I-1-3 was obtained by suction filtration with a yield of 91%.
[0218] 1 H NMR(300MHz,DMSO-d6)δ(ppm)8.85(brs,1H),7.64-7.69(m,2H),7.48-7.52(m,1H), 7.00(d,1H,J=7.62Hz), 6.59(m,1H), 3.80-3.85(m,2H), 3.68(m,3H), 3.58(m,1H), 3.14(m,1H), 2.99(m,2H), 2.74-2.79(m, 2H), 1.59 (m, 1H), 1.29-1.32 (m, 1H), 1.19-1.13 (m, 2H), 0.82 (d, 6H).
[0219] ESI m / z: 475.9[M+H] + .
[0220] Compound I-1-3 was obtained according to the above preparation process, and it was detected at a temperature of 20~25℃. The X-ray powder diffraction pattern of this crystal form is shown in the a...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap