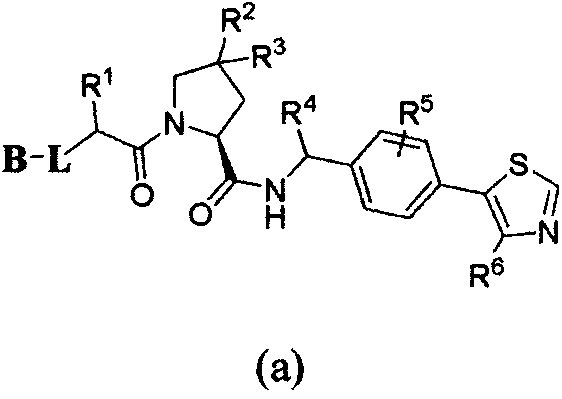
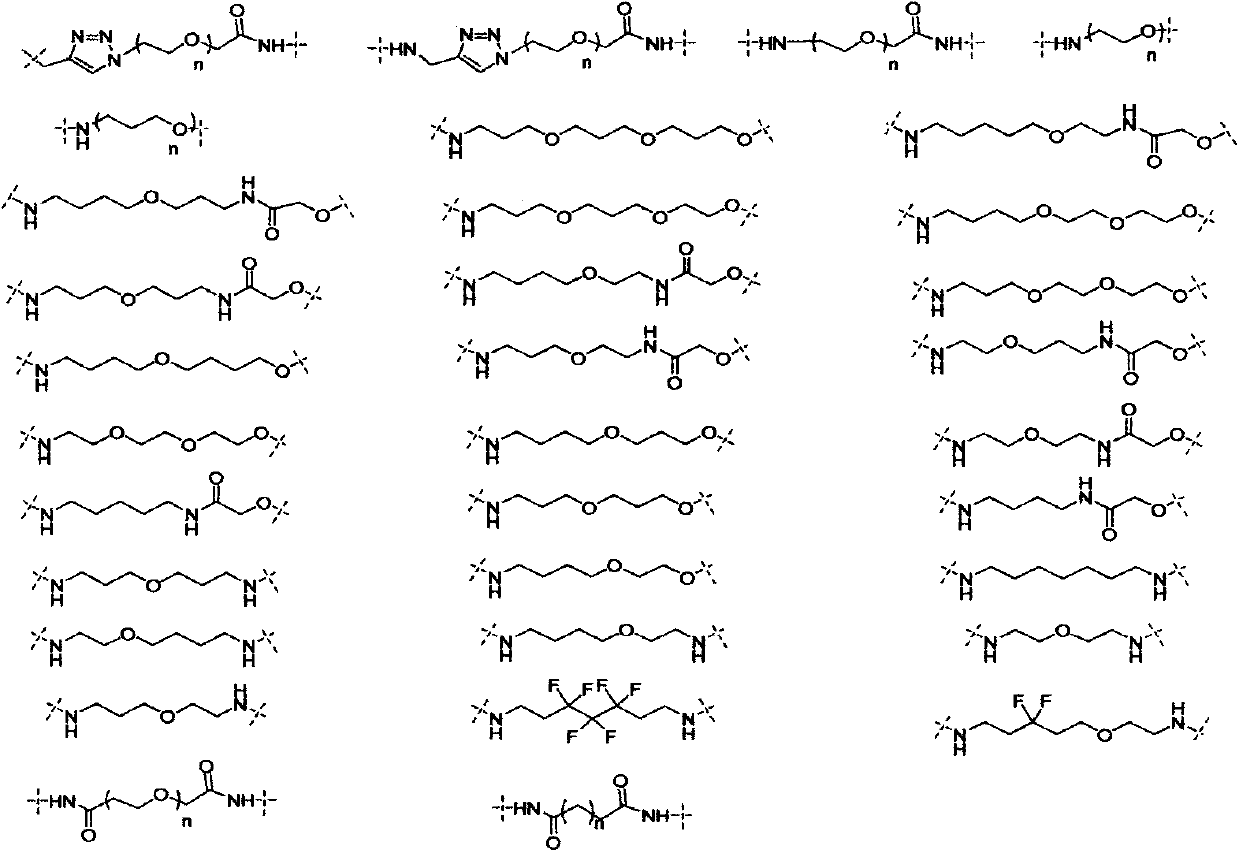
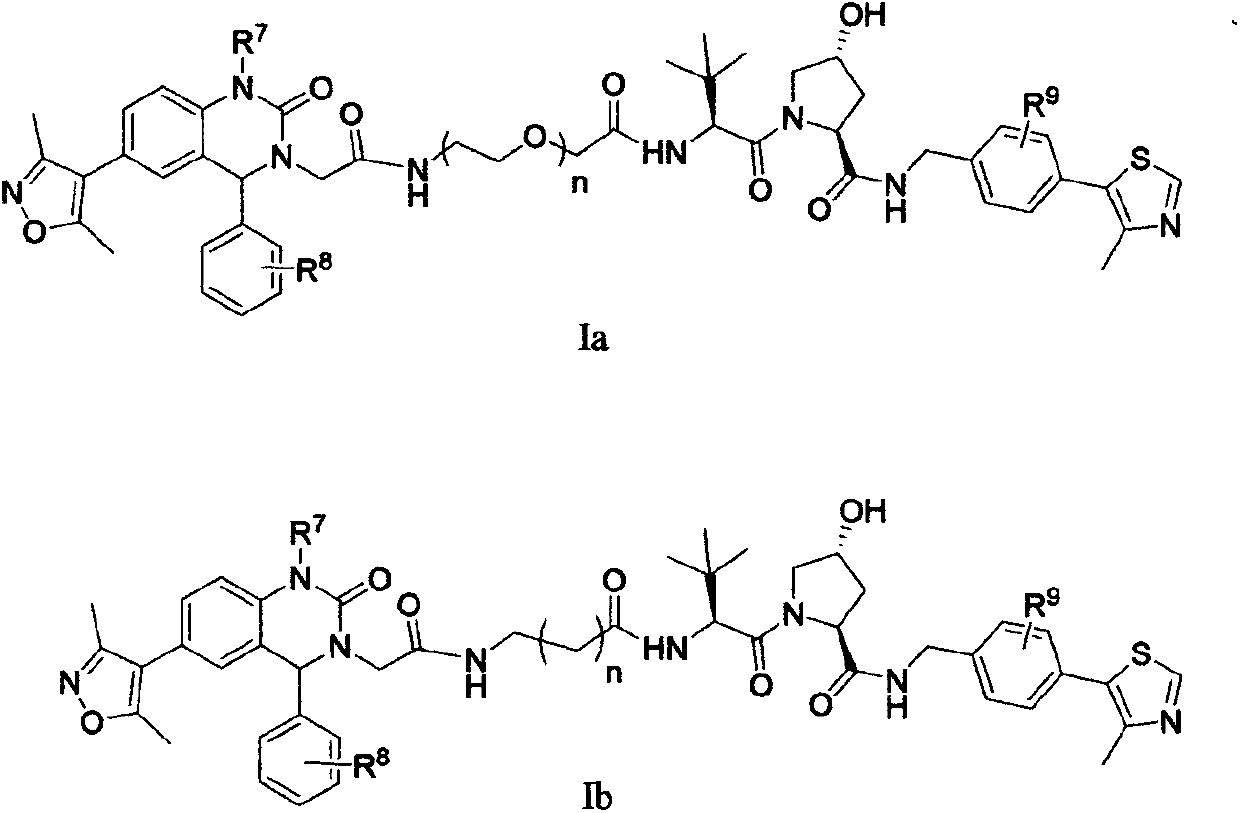
Bifunctional molecules for induction of BET degradation based on VHL ligand and BET inhibitor, preparation and application thereof
A technology of -CH3 and CHF2, applied in the field of bifunctional molecules based on VHL ligand and BET inhibitors to induce BET degradation and its preparation and application, can solve the problems of thrombocytopenia, unsatisfactory anti-tumor effect of drugs, and toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: (2S, 4R)-1-((2S)2-(2-(2-(2-(6-(3,5-lutidine-4-yl)-1-methyl- 2-oxo-4-phenyl-1,4-dihydroquinazolin-3(2H)-yl)acetamido)ethoxy)acetamido)-3,3-dimethylbutyryl)-4 -Hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (Ia-1), its structural formula is as follows:
[0082]
[0083] Step 1) 2-Hydroxyethyl-4-methylbenzenesulfonate (1a)
[0084]
[0085] Dissolve ethylene glycol (3.91g, 62.95mmol) in 5mL of pyridine, add p-toluenesulfonyl chloride (6g, 31.47mmol) in batches, stir at room temperature for 4 hours, add 6mol / L hydrochloric acid (40mL), wash with ethyl acetate Extract, wash with saturated brine, collect the organic layer, dry over anhydrous sodium sulfate, evaporate the organic solvent under reduced pressure, and purify the residue by silica gel column chromatography using petroleum ether / ethyl acetate (V / V=20 / 1-10 / 1) was eluted to obtain a colorless liquid weighing 2 g with a yield of 29.39%.
[0086] 1 H NMR (300MHz, CDCl 3 )δ7.8...
Embodiment 2
[0108] Example 2: Preparation of (2S, 4R)-(1-((2S)-2-(2-(2-(2-(4-((6-(3,5-dimethylisoxazole-4 -yl)-1-methyl-2-oxo-4-phenyl-1,4-dihydroquinazol-3(2H)-yl)acetamido)ethoxy)ethoxy)acetamido)- 3,3-dimethylbutyryl)-4-hydroxyl-N-(4-(4-methylthiazol-5-yl)benzyl)-2-pyrrolidinecarboxamide (Ia-2), its structural formula is as follows :
[0109]
[0110] Synthetic steps are with embodiment 1
[0111] MS (ESI, m / z): 947.10 [M-H] -
Embodiment 3
[0112] Example 3: Preparation of (2S, 4R)-(1-((2S)-2-(2-(2-(2-(2-(4-((6-(3,5-dimethylisoxan Azol-4-yl)-1-methyl-2-oxo-4-phenyl-1,4-dihydroquinazol-3(2H)-yl)acetamido)ethoxy)ethoxy)ethyl Oxy)acetylamino)-3,3-dimethylbutyryl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)-2-pyrrolidinecarboxamide (Ia -3), its structural formula is as follows:
[0113]
[0114] Synthetic steps are with embodiment 1
[0115] MS (ESI, m / z): 991.10 [M-H] -
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com