Application of a long-acting compound in the preparation of medicines
A compound and compound structural formula technology, applied to the application field in the preparation of medicines, can solve the problems of limiting the long-term effect of depression and the like, and achieve the effect of long drug effect time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1: compound preparation method
[0097]
[0098] Step 1: Starting from o-trifluoromethylbenzonitrile, according to the classic ketamine drug synthesis method Calvin Stevens method (preparation route as shown above) to obtain compound D 1 ;
[0099] Step 2: compound D 1 (2.57g, 10mmol) was added into 60ml THF, triethylamine (2.7mL, 20mmol) and Boc 2 O (3.32g, 15mmol), refluxed for 6h, cooled, spin-dried, and passed through a silica gel column to obtain compound E 1 3.25 g, 91% yield. 1 H NMR (300MHz, CDCl 3 ): δ7.80(d, J=8.1Hz, 1H), 7.66(d, J=7.8Hz, 1H), 7.49(t, J=7.5Hz, 1H), 7.22(t, J=7.5Hz, 1H ),5.76(s,1H),4.30(s,1H),3.46-3.42(m,1H),2.45–2.36(m,1H),2.35-2.22(m,1H),1.76–1.60(m,6H ),1.21(s,9H). 13 C NMR (75MHz, CDCl 3 ): δ208.9, 151.7, 149.0, 148.6, 136.1, 133.8, 132.6, 130.5, 129.0, 128.1, 128.0, 127.9, 127.8, 121.8, 121.7, 88.9, 80.8, 73.7, 40.0, 29.4, 28.3, 27.
[0100] Step 3: Compound E 1 (2.14g, 6mmol) was added to dry 50ml THF, under argon ...
Embodiment 2
[0103] Embodiment 2: activity test method:
[0104] 1. Forced swimming experiment
[0105] Transfer mice to the laboratory 1 h before the forced swim test (FST). The tests were performed under normal lighting conditions and monitored by a digital video camera. During the test, the mice were placed individually in transparent glass cylinders (28.5 cm high, 14 cm in diameter) containing 20 cm of water (23±1° C.). On the first day, mice were trained for 6 min before being removed from the cylinder. On the second day, mice were administered vehicle (physiological saline) control group, HNK, CF3, and then their immobility time was tested after 1 hour and 7 days, wherein immobility time refers to passive floating without other movements. During the last 4 min of the entire 6-min swim test, immobility time was recorded by the Nodus system's EthoVision XT (Noldus, Netherlands) (Castagné et al., 2011, Hajmirzaian et al., 2014, Porsolt et al., 1977). After every two to three trials,...
Embodiment 3
[0118] Embodiment 3: experimental result
[0119] 1. Depression-like behavior test results in mice:
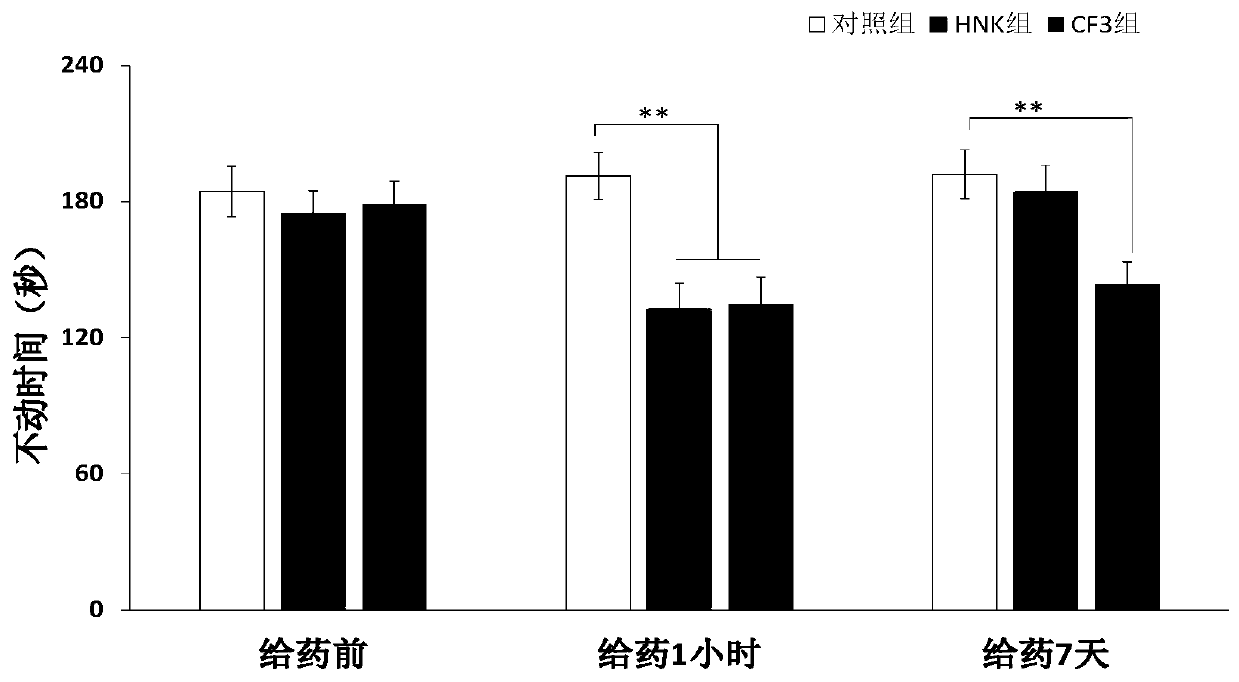
[0120] like figure 1 Shown:
[0121] Depression-like behaviors were measured by the forced swim test. Mice received 10 mg of HNK, CF3 by intragastric administration, and immobility time was measured after 1 hour and 7 days. Percentage of immobility time is expressed as mean ± SEM *p<0.05, **p<0.0 compared to preactivity. N=8 saline per group:
[0122] Saline mice; HNK: 2R,6R-hydroxynorchloramine-treated mice; CF3, CF3-treated mice.
[0123] 2. Differential protein analysis:
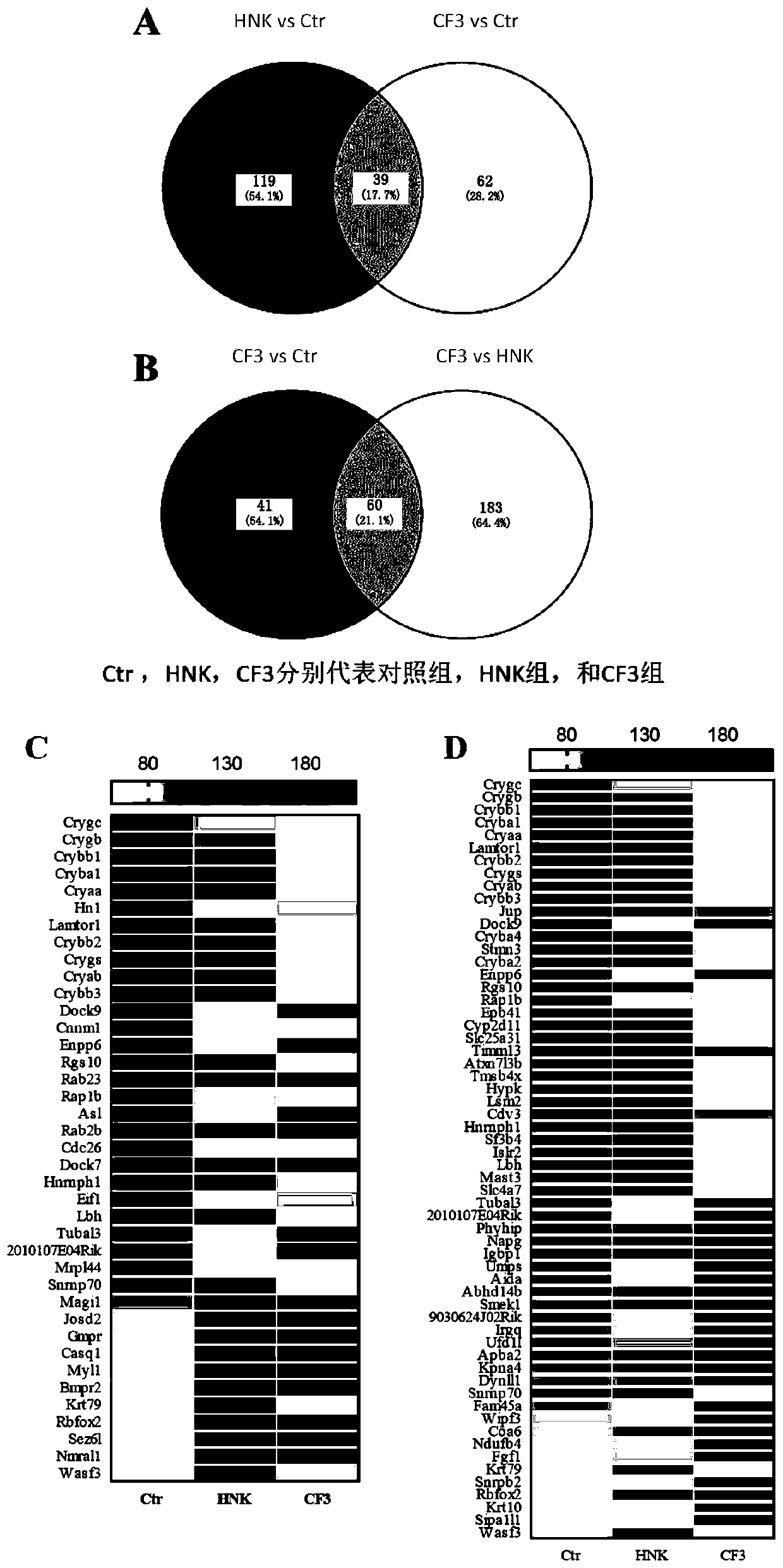
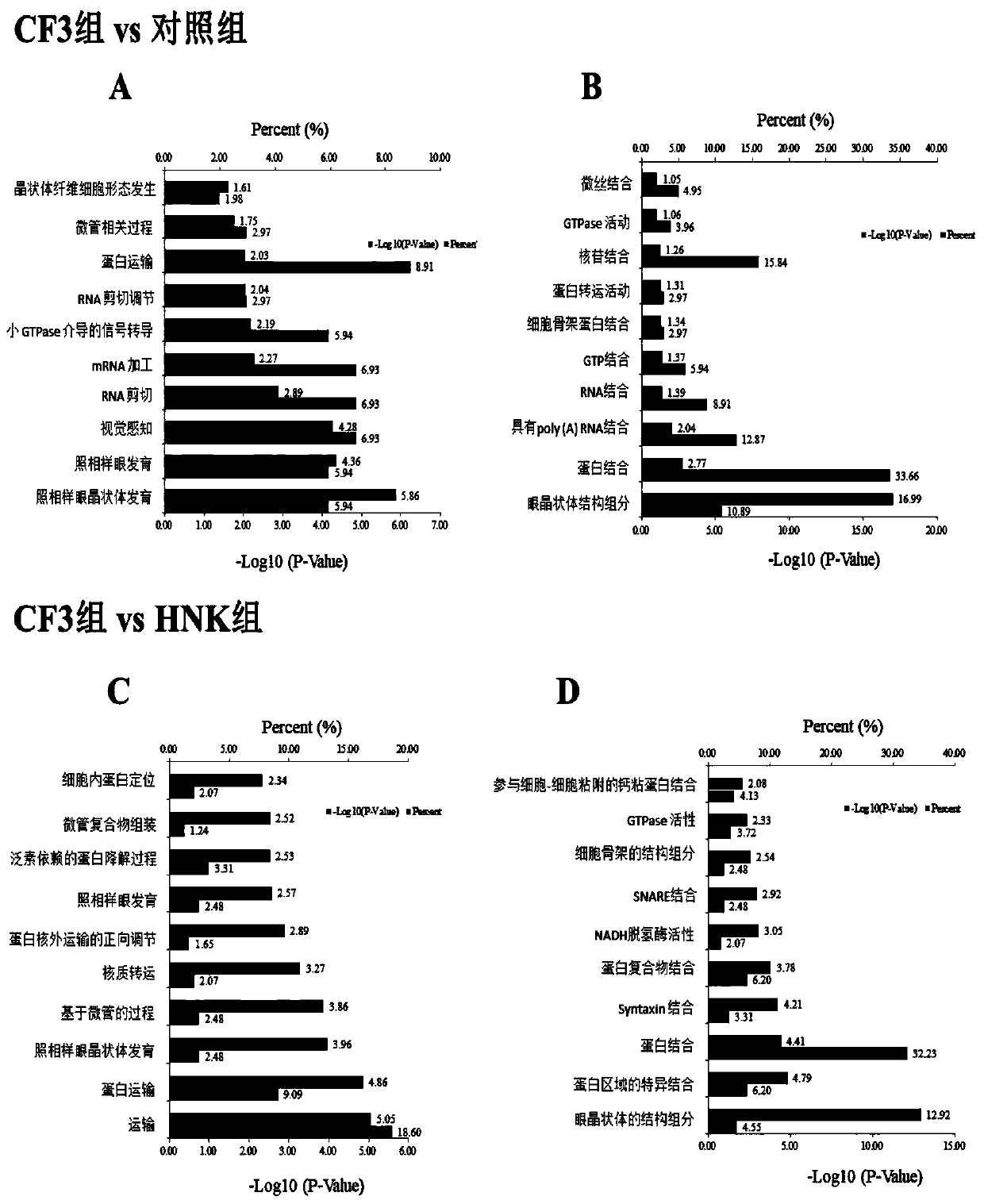
[0124] like figure 2 Shown:
[0125] A, with 0.833>Abundance Ratio>1.2 as the critical point, a Ratio value higher than 1.2 is defined as an up-regulated protein, and a Ratio value lower than 0.833 is defined as a down-regulated protein. Compared with the control group, there were 101 differentially expressed proteins in the CF3 treatment group, among which 39 differentially expressed proteins ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap