A purification method of recombinant human glucagon-like peptide-1 analog
A technique for glucagon and a purification method, which is applied in the field of purification of recombinant human glucagon-like peptide-1 analogs, and can solve problems such as slow flow rate, inability to carry out industrial scale-up production, and increased pressure in the chromatography process. Achieve the effect of low production cost and improve the feasibility of enlarged production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Recombinant expression of precursor fusion protein in Escherichia coli was pretreated and denatured, captured by anion chromatography and digested with enterokinase to remove the tagged protein to obtain GLP-1 analogs
[0041] Recombinant Expression of DsbC-DDDK-Arg in Escherichia coli by DNA Recombination Technology 34 GLP-1(7-37) fusion protein, the recombinant cells were fermented and centrifuged to collect the cells, the recombinant cells were used to extract the inclusion bodies and denatured, then use anion chromatography to capture the fusion protein, and use 200mM NaCl for linear gradient elution to collect the main peak . After capturing the fusion protein, adjust the pH to 8.0, and add recombinant enterokinase at a ratio of 10 IU / mg for enzyme digestion.
Embodiment 2
[0042] Example 2: Purification using Source 30Q media
[0043] This embodiment provides a purification method for GLP-1 analogues, specifically:
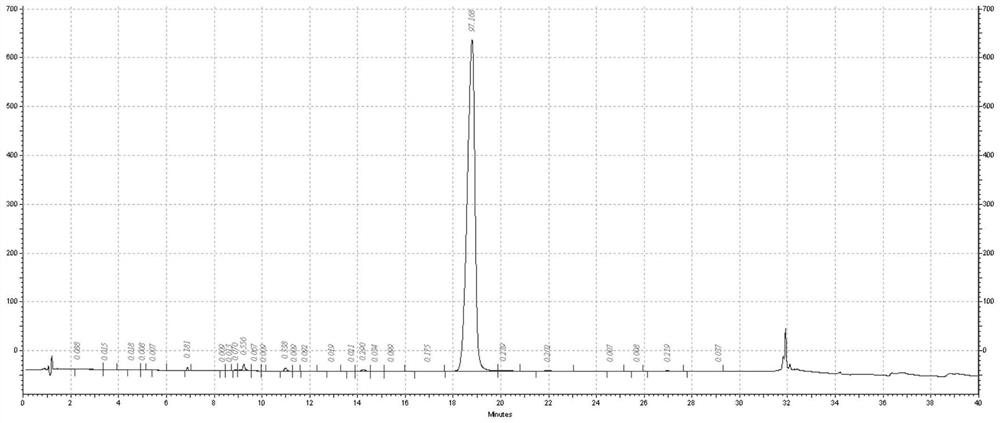
[0044] (1) Dilute hydrochloric acid is added to the feed solution after enzymatic digestion in Example 1, and the pH is adjusted to 5.8, and the isoelectric point precipitation is carried out. After standing overnight, the precipitate is collected by centrifugation, which is Arg 34 GLP-1(7-37), the purity is about 80%, the recovery rate is more than 90%;
[0045] (2) Resuspend the precipitate obtained in step (1) in 20mM Tris and adjust the pH to 7.0 with sodium hydroxide to make Arg 34 GLP-1(7-37) was redissolved, adding purified water to adjust the conductance to no more than 6ms / cm 2 , filtered and clarified;
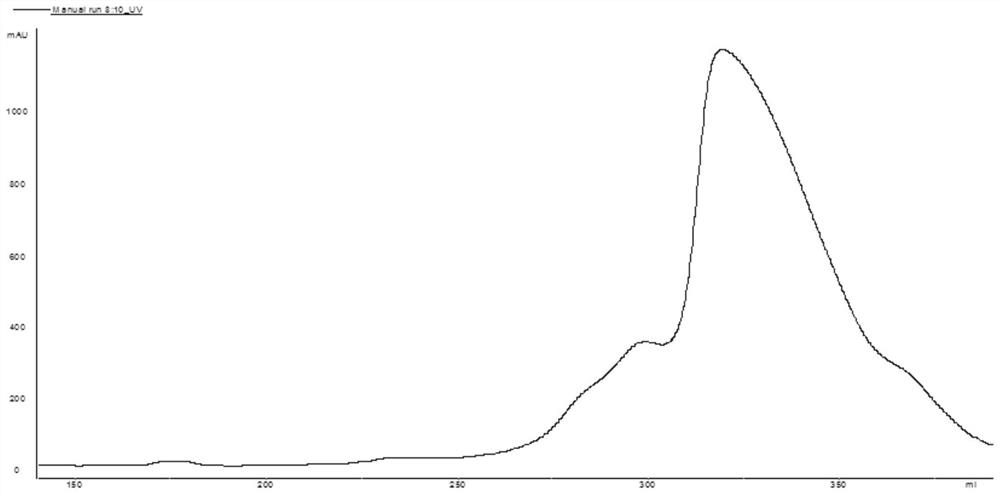
[0046] (3) Take the feed liquid obtained in step (2) and apply it to Source 30Q filler, the loading amount is 20g / L gel. After the sample is loaded, wash 3 column volumes with pH7.0 5mMTris-HCl, and then wash with pH7.0...
Embodiment 3
[0049] Example 3: Purification using UniGel 30Q media
[0050] This embodiment provides a purification method for GLP-1 analogues, specifically:
[0051] (1) Dilute hydrochloric acid is added to the feed liquid after enzymatic digestion in Example 1, the pH is adjusted to 5.2, and the isoelectric point precipitation is carried out. After standing overnight, the precipitate is collected by centrifugation, which is Arg 34 GLP-1(7-37), the purity is about 78%, and the recovery rate is more than 91%;
[0052] (2) Resuspend the precipitate obtained in step (1) in 20mM Tris and adjust the pH to 9.0 with sodium hydroxide to make Arg 34 GLP-1(7-37) was redissolved, adding purified water to adjust the conductance to no more than 6ms / cm 2 , filtered to clarify.
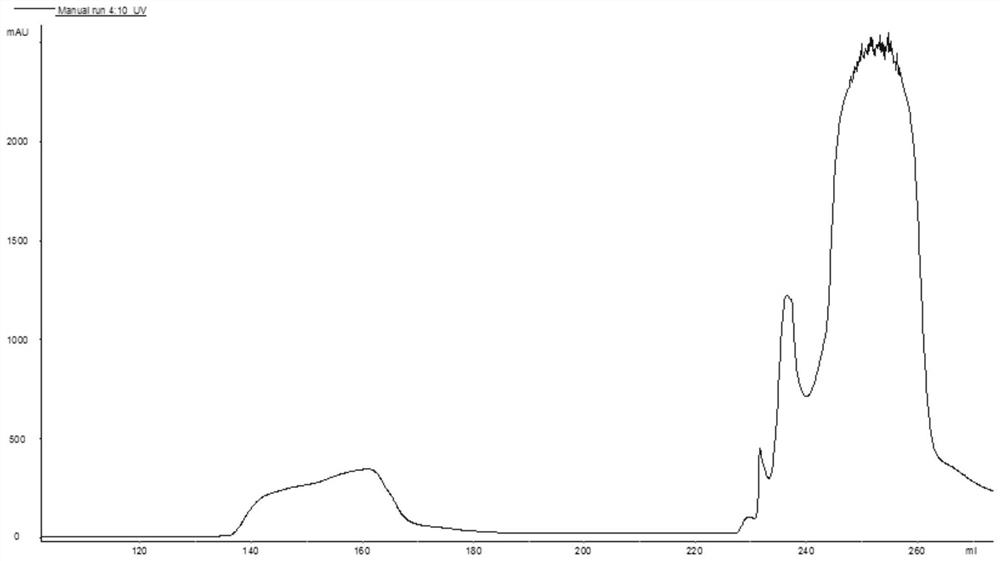
[0053] (3) Take the feed liquid obtained in step (2) and apply it to UniGel 30Q filler, the loading amount is 20g / L gel. After the sample loading is completed, wash 3 column volumes with pH9.0 50mMTris-HCl, then use pH9.0, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com