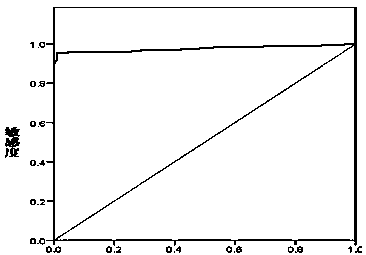
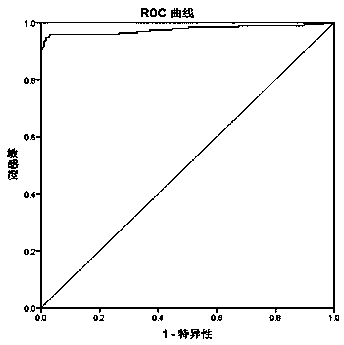
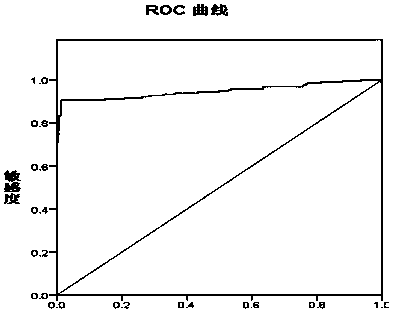
Novel coronavirus IgM/IgG antibody chemiluminiscence method detection kit
A technology of chemiluminescence and detection kits, which is applied in the direction of measuring devices, scientific instruments, instruments, etc., and can solve problems such as many steps, high requirements for venues and equipment, and long detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0105] The preparation of FITC-labeled novel coronavirus antigen comprises the following steps,
[0106] 1) Mix FITC and the novel coronavirus antigen at a molar ratio of 4:1, and react at 37°C for 12 hours in the dark;
[0107] 2) Dialyze the labeling solution completed in step 1) with 0.01M PBS at 2-8°C for 24 hours, add an equal volume of glycerol, and store at -20°C.
[0108] Among them, the 2019-nCoV antigens are 4 synthetic polypeptides mixed in the same mass ratio; the amino acid sequences of the 4 synthetic polypeptides are shown in Table 1.
[0109] The 20-fold concentrated lotion includes 58g / L disodium hydrogen phosphate, 5.92g / L sodium dihydrogen phosphate, 180g / L NaCl, 10mL / LTween-20 and 2% Proclin300 in mass concentration.
[0110] Negative control and positive control reagents, wherein the negative control, preparation method: add the buffer solution containing bovine serum albumin to 1% volume concentration of ProClin TM 300. Aliquot, label and store at 2-8...
Embodiment 1
[0112] The preparation of the anti-FITC antibody-coated plate used in Example 1 and Example 2 includes the following steps, diluting the anti-FITC antibody to 5 μg / mL with 0.02M phosphate buffer solution, and adding it to a 96-well white opaque plastic microwell plate at the same time , 2-8°C for 16-24 hours; discard the liquid in the well, wash the plate with pH 7.4 PBS buffer, then add phosphate buffer containing 0.5% BSA to seal the microwell plate, 2-8°C Seal for 16-24 hours; discard the liquid in the hole, dry it at 37°C for 20-24 hours; put it in an aluminum foil bag, add a desiccant, seal it, label it, and store it at 2-8°C.
[0113] The preparation of the anti-FITC antibody-coated plate used in Example 3 and Example 4 includes the following steps: Dilute the anti-FITC antibody to 5 μg / mL with 0.02M phosphate buffer, and add it to a 96-well white opaque plastic microwell plate at the same time , coat at 37°C for 2 hours; discard the liquid in the well, wash the plate wi...
Embodiment 2
[0189] A new coronavirus IgM antibody chemiluminescence detection kit, comprising the above-mentioned anti-FITC antibody coated plate, FITC-labeled new coronavirus antigen, alkaline phosphatase-labeled mouse anti-human IgM monoclonal antibody, 20 times concentrated washing solutions, negative controls, and positive controls.
[0190] The preparation method of alkaline phosphatase-labeled mouse anti-human IgM monoclonal antibody is as follows:
[0191] A. Alkaline phosphatase (ALP) activation
[0192] 1) Prepare 10mg / mL ALP solution;
[0193] 2) Prepare 12.8 mg / mL sodium periodate NaIO 4 solution;
[0194] 3) Mix the solution prepared in step 1) and step 2) at a volume ratio of 1:1, and react at 2-8°C in the dark for 30 minutes;
[0195] 4) Prepare an aqueous solution of ethylene glycol with a concentration of 20 μL / mL, mix it with the same volume as the solution prepared in the above step 3), react at room temperature and avoid light for 20 minutes, the activation is compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com