Compounds for treatment of cardiac arrhythmias and heart failure
A technology for compounds and hydrates, which can be used in the active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc., and can solve problems such as controversial mechanisms of action.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0206] Certain embodiments are described below in the following numbered items:
[0207] 1. The compound of formula I or its stereoisomer, tautomer, pharmaceutically acceptable salt, hydrate or solvate:
[0208]
[0209] in:
[0210] -N(R 1 ) and R A with R B together form a 5- or 6-membered heteroaliphatic or heteroaryl ring; R C is H, aliphatic or -O-C(O)-aliphatic, and R D is a substituted aliphatic group or -Y-X-(CH 2 ) m -N(R 4 )R 5 , or R C and R D together form a 5- or 6-membered heteroaliphatic or heteroaryl ring; R E is H, aliphatic, -O-aliphatic, or -O-C(O)-aliphatic; Q is N or C-R 3 ; 1 and R 2 is independently H or an aliphatic group; R 3 is H, aliphatic or -O-aliphatic; R 4 and R 5 is independently H, aliphatic, or aryl; X is absent or N(H), O, C(O), -S(O 2 )O-,-OS(O 2 )-, -P(O)(OH)O-, -OP(O)(OH)-, -N(H)-C(H)(CF 3 )-or-C(H)(CF 3 )-N(H)-; Y is-(CH 2 ) n - or a bivalent azole ring; and m and n are independently integers from 1 to 10, wherei...
Embodiment 1
[0246] Synthesis of Representative Compounds
[0247]
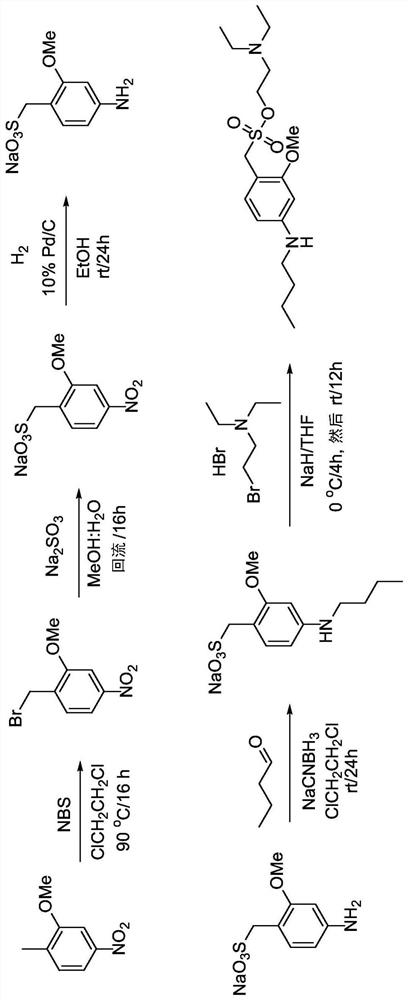
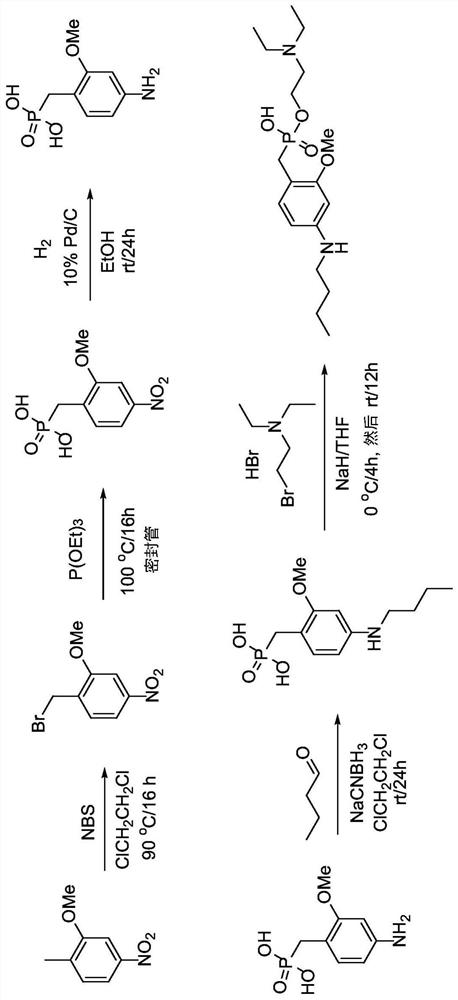
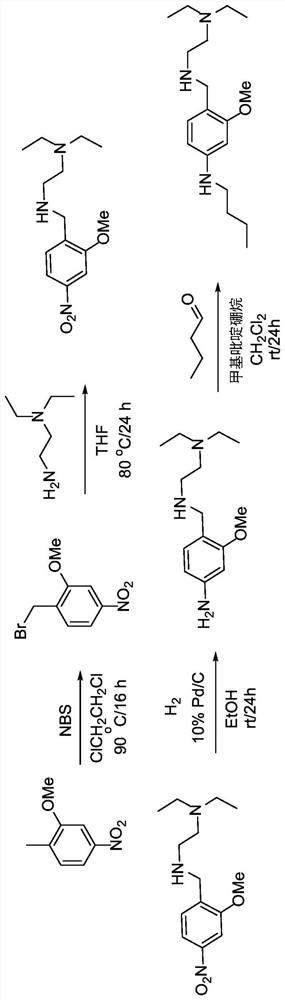
[0248] Synthesis of 1-(bromomethyl)-2-methoxy-4-nitrobenzene (233). 2-Methoxy-1-methyl-4-nitrobenzene (20.2 g, 118 mmol) was dissolved in 500 ml anhydrous DCE and Ar was bubbled through the mixture for 5 min. N-bromosuccinimide (23.2 g, 129 mmol) was added to the flask and stirred for 5 minutes. Benzoyl peroxide (1.89 g, 5.85 mmol) was added to the flask and stirred for 5 minutes. The flask was heated to 90°C and a lamp was tilted towards the flask for direct illumination. The reaction mixture was stirred under argon for 24 hours. The mixture was then cooled to room temperature. Pour the mixture into a one-liter separatory funnel, and 2 O (100ml) wash. use CH 2 Cl 2 (3 x 50ml) to extract the aqueous layer. The combined organic extracts were washed with brine (250ml), washed with Na 2 SO 4 Dry, filter, and concentrate under vacuum. The crude mixture was separated by flash column chromatography on silica gel,...
Embodiment 2
[0352] Compound Characterization
[0353] Ca 2+ Spark screening. By a modified collagenase method (Li et al. J.Vis.Exp.2014, 87:351357; Li et al., Circ Res 2012, 110:465-470; van Oort et al., Hypertension 2010,55:932-8) RyR2 from 20±4 weeks old R176Q / + Single ventricular myocytes isolated from mouse hearts. Ca loading on ventricular myocytes 2+ indicator Fluo-4-AM (2 mol / l) and treated with 100 nM isoproterenol (ISO) to induce spontaneous Ca 2+ Spark activity increased. Preloaded cardiomyocytes were exposed to 500 nM RyR2 inhibitor or left untreated for 1 hour (37°C). Inhibitors were reconstituted as 0.1 M working stocks in DMSO, followed by serial dilutions to obtain the desired 500 nM concentration. By measuring Ca 2+ Spark frequency (CaSpF), RyR2 activity was assessed in preloaded ventricular myocytes to determine whether RyR2 inhibitors make excess SR Ca 2+ release normalization. Ca was recorded using a LSM880 Zen Black confocal microscope (Carl Zeiss) at 500 Hz ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap