Application of securinine linked dimeric compound SN3-L6 or pharmaceutical salt thereof in preparation of anti-leukemia drugs
A technology of SN3-L6 and hagiline chain, applied in the field of medicine, can solve the problems of limited validity period, improper platelet preservation method, and insufficient supply of platelets, etc., and achieve the effect of inhibiting cell proliferation and improving medicinal prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
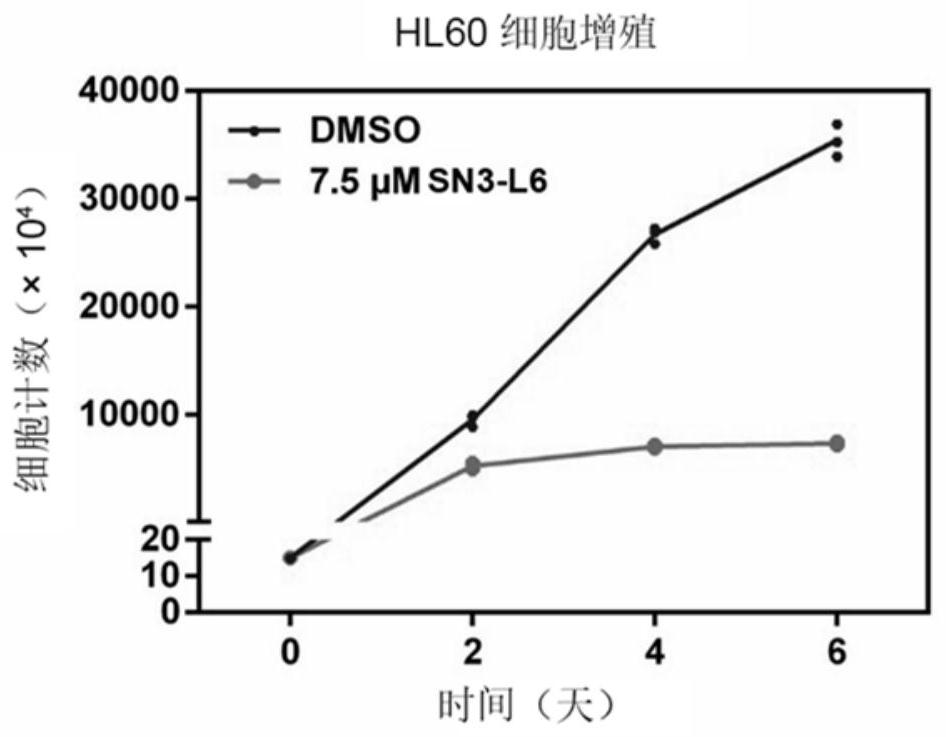
[0041] Example 1 Inhibitory effect of SN3-L6 on proliferation of leukemia cell HL60
[0042] (1) Experimental method: Take HL60 cells (purchased from Sun Yat-Sen University Cell Bank) in logarithmic phase growth, inoculate in 25cm 2 Cell culture flask, 15,000 / mL, 10mL / bottle. Add the final concentration of 7.5μM SN3-L6 and an equal volume of DMSO (dimethyl sulfoxide, the final concentration of which should be controlled below 0.1% (v / v)) for treatment, put them into a constant temperature cell incubator, and change them every two days solution and add medicine once, cell counting is carried out every two days (dilution culture is carried out when the cell density is greater than 500,000), and the cell growth curve is drawn, such as figure 1 shown.
[0043] (2) Test results: SN3-L6 can significantly inhibit the proliferation of HL60 cells.
Embodiment 2
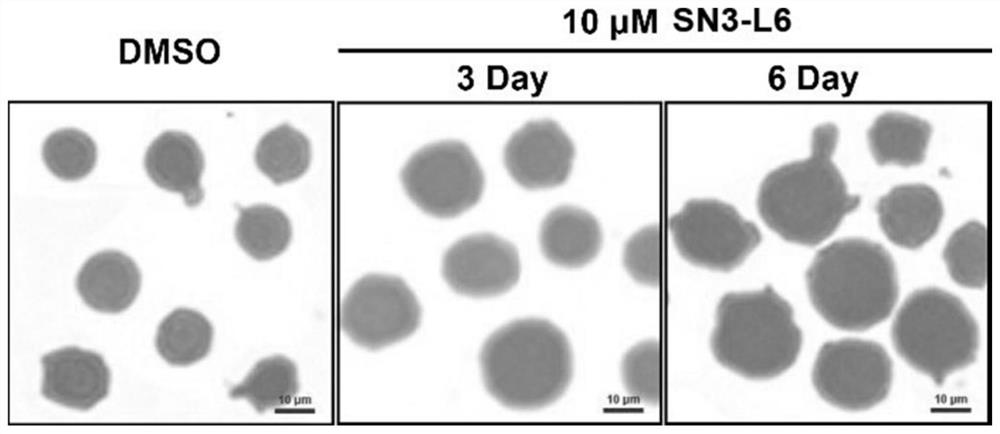
[0044] Example 2 Wright's-Giemsa staining of HL60 cells after SN3-L6 treatment
[0045] (1) Experimental method: Take HL60 cells grown in logarithmic phase, inoculate in 6-well plate, 50,000 / well, 2mL / well, add SN3-L6 with a final concentration of 10 μM and an equal volume of DMSO (final concentration control (below 0.1% (v / v)) for 3 days and 6 days respectively, collect the cell suspension and centrifuge on the 3rd or 6th day, discard the supernatant, then add PBS (PBS buffer, pH 7.4) to resuspend and centrifuge again Collect the cell pellet and wash repeatedly three times to collect the cells. Finally, 200 microliters of PBS was added to resuspend the cells, spread on a glass slide soaked in paraformaldehyde and dried in the air, so that the cells were evenly distributed in a single layer on the glass slide. Add 1000 microliters of Giemsa-Wright's staining solution A to the cell area on the slide, and at the same time gently blow the staining solution with the ear washing b...
Embodiment 3
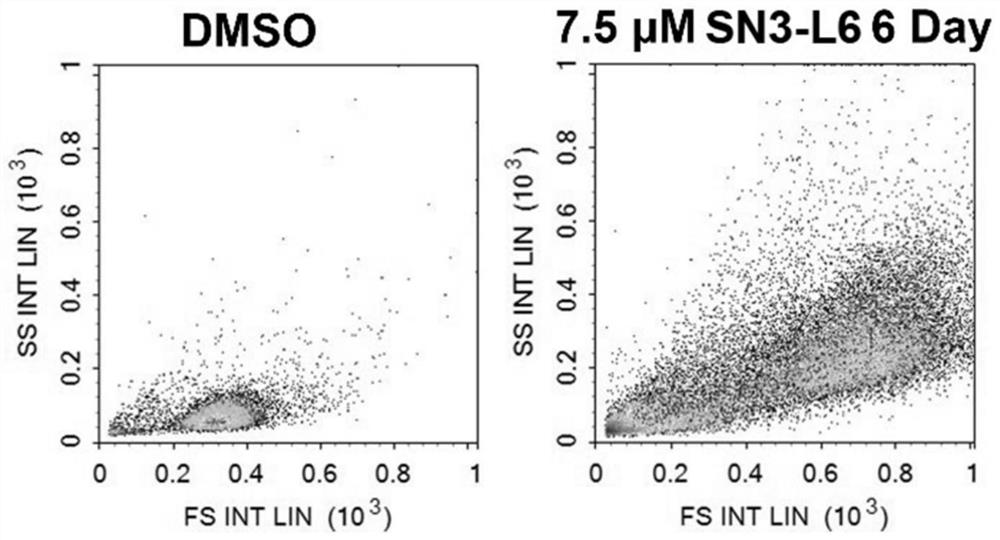
[0047] Example 3 Flow cytometric detection of HL60 cells after SN3-L6 treatment
[0048] (1) Experimental method: HL60 cells in the logarithmic growth phase were inoculated in 6-well plates, 100,000 cells / well, and SN3-L6 with a final concentration of 7.5 μM and an equal volume of DMSO were added (the final concentration was controlled at 0.1% ( v / v) below) after treating HL60 cells for 6 days, collect the cells by centrifugation, wash with PBS three times and then detect the cell signal by flow cytometry, the results are as follows image 3 shown.
[0049] (2) Test results: After 7.5 μM SN3-L6 was applied to HL60 cells, the FSC value on the abscissa moved significantly to the right, indicating that the cells were significantly larger, and the SSC value on the ordinate moved significantly upward, indicating that the complexity of the granules inside the cells also increased significantly.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap