Pharmaceutical composition for treating cerebral apoplexy and acute cerebral infarction
A composition and cerebral apoplexy technology, applied in the field of biomedicine, can solve the problems of undetectable Apelin prototype drug, short half-life, hindering the development of Apelin clinical therapeutic drugs, etc., and achieve the effect of reducing the volume of cerebral infarction and reducing neurological dysfunction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: The solid-phase synthesis of 2-(4-chlorophenyl)-2,2-difluoroacetic acid-QRPRLSHKGPMPF
[0023] (1) Weigh 1mmol of 2-cl-Trt resin in a solid-phase synthesizer, add 15mL of anhydrous dichloromethane (hereinafter referred to as DCM), place on a shaker and shake for 5min to fully swell the 2-Cl-Trt resin ;
[0024] (2) DCM is removed from the solid-phase synthesizer equipped with 2-Cl-Trt resin with ear washing ball;
[0025] (3) Dissolve 0.75 mmol of Fmoc-Phe in 10 mL of anhydrous DCM, add 0.75 mmol of DIPEA, then transfer to the above-mentioned solid-phase synthesizer, add 0.75 mmol of DIPEA, and react at room temperature for 1 h;
[0026] (4) Sealing: remove the reaction liquid in the solid-phase synthesizer with ear washing balls, then wash with 10 mL of anhydrous DCM, each time for 1 min, and wash 5 times in total, add the prepared volume ratio of anhydrous DCM: DIPEA: methanol =17:1:2 solution 20mL, react at room temperature for 10min;
[0027] (5) Re...
Embodiment 2
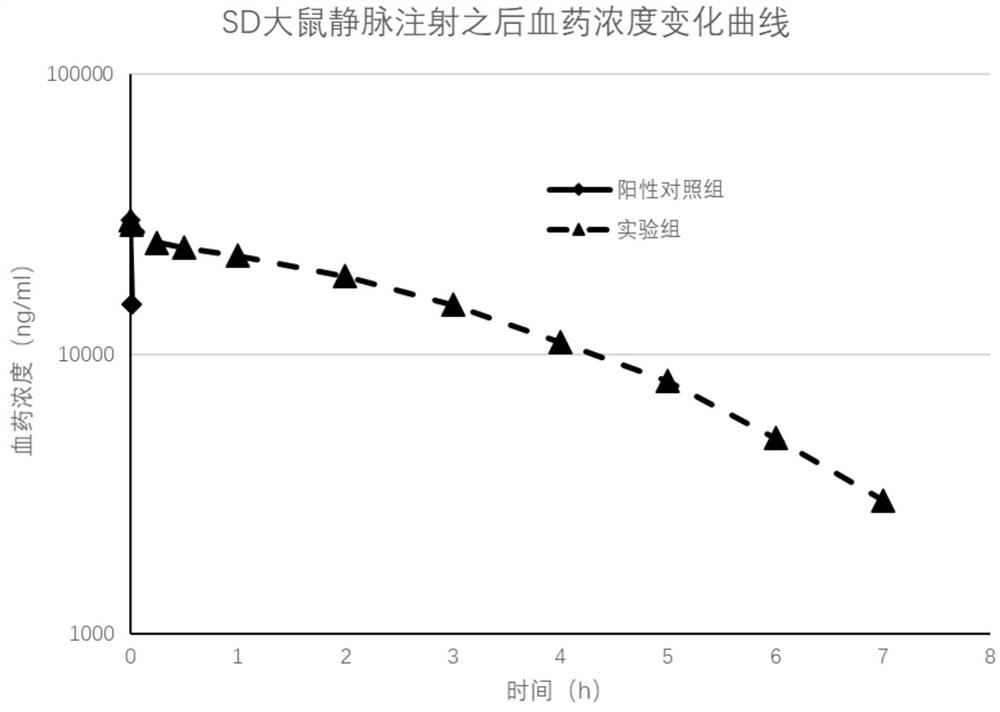
[0032] Embodiment 2: Experiment of the biological half-life of the target active peptide in rats
[0033] (1) Experimental animal information
[0034] SPF grade SD rats, 16 males, weighing 190g to 210g, were provided by the Experimental Animal Center of Wenzhou Medical University.
[0035] (2) Dosing regimen and plasma sample collection and processing
[0036] Intravenous injection, the dosage was 10mg / kg, the rats were randomly divided into positive control [Pyr1]-Apelin-13 solution group, experimental group 2-(4-chlorophenyl)-2,2-di Fluoroacetic acid-QRPRLSHKGPMPF solution group, a total of 2 groups (n=8), fixed rats, administered through the tail vein. At different time points after administration, 0.2 mL of whole blood was collected through the jugular vein cannula and placed in a 1.5 mL LEDTA-2K anticoagulant centrifuge tube. ) to seal the tube for smooth collection at the next time point. The blood sample was centrifuged at 4000g for 5 minutes at 4°C, the plasma was ...
Embodiment 3
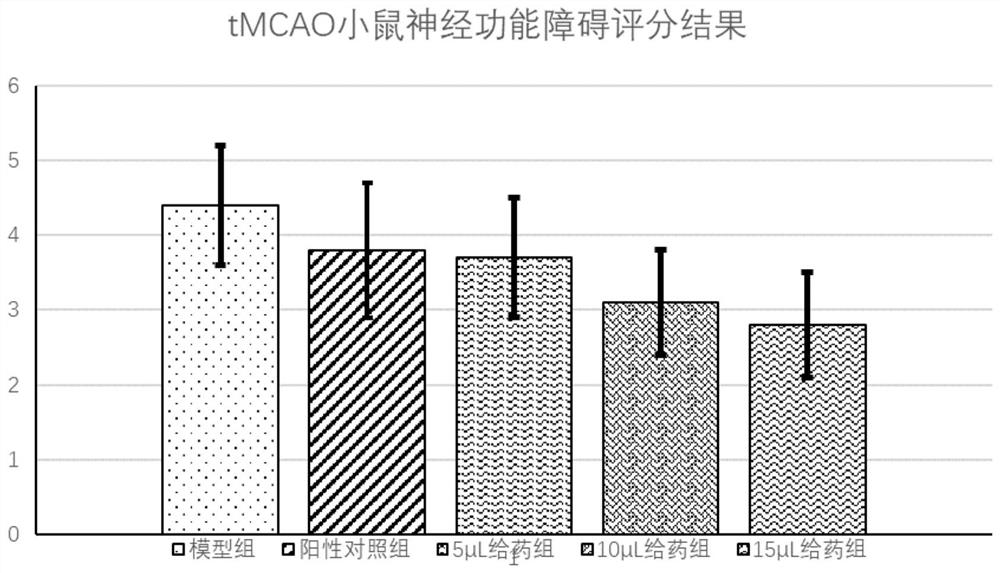
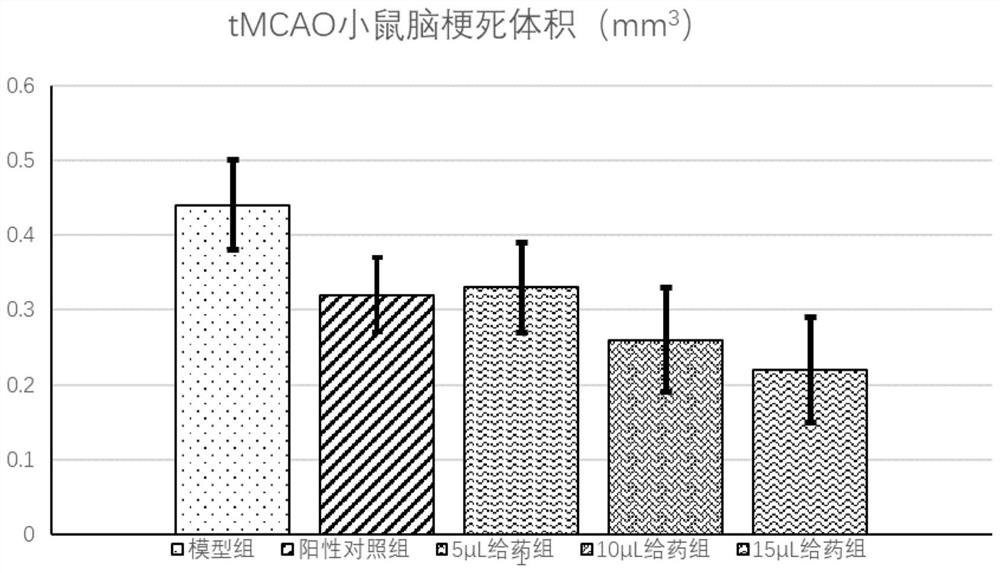
[0042] Example 3: The treatment of 2-(4-chlorophenyl)-2,2-difluoroacetic acid-QRPRLSHKGMPPF for cerebral apoplexy and cerebral infarction
[0043] 3.1 Experimental materials
[0044] Experimental Animals and Breeding
[0045] 2-3 months old, male C57BL / 6J mice, 24-28g, provided by Experimental Animal Center of Wenzhou Medical University. Raising conditions: the room temperature is between 22-24° C., the humidity is between 40-70%, the lighting time is 12 hours alternately between light and dark, and free to drink and eat.
[0046] 3.2 Modeling of tMCAo mice: Mice were fasted for 8-10 hours before surgery and had free access to water. Mice were anesthetized by intraperitoneal injection of chloral hydrate (350 mg / kg). According to the Longa method, the right middle cerebral artery occlusion (middle cerebral artery occlusion) in the monofilament lumen was prepared with 6-0 silica gel-coated nylon monofilament suture (Doccol Corp). , MCAO) model. After 60 minutes of ischemia, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com